Abstract
A range of diseases is associated with amyloid fibril formation. Despite different proteins being responsible for each disease, all of them share similar features including beta-sheet-rich secondary structure and fibril-like protein aggregates. A number of proteins can form amyloid-like fibrils in vitro, resembling structural features of disease-related amyloids. Given these generic structural properties of amyloid and amyloid-like fibrils, generic inhibitors of fibril formation would be of interest for treatment of amyloid diseases. Recently, we identified five outstanding inhibitors of insulin amyloid-like fibril formation among the pool of 265 commercially available flavone derivatives. Here we report testing of these five compounds and of epi-gallocatechine-3-gallate (EGCG) on aggregation of alpha-synuclein and beta-amyloid. We used a Thioflavin T (ThT) fluorescence assay, relying on halftimes of aggregation as the measure of inhibition. This method avoids large numbers of false positive results. Our data indicate that four of the five flavones and EGCG inhibit alpha-synuclein aggregation in a concentration-dependent manner. However none of these derivatives were able to increase halftimes of aggregation of beta-amyloid.
Keywords: Amyloid, Fibril, Inhibitor, Protein aggregation, Flavone, Amyloid beta, Alpha-synuclein
Introduction
Amyloid fibril formation is related to a number of fatal neurodegenerative disorders, such as Alzheimer’s and Parkinson’s diseases and transmissible spongiform encephalopaties. One of the strategies for pharmacological intervention is to inhibit protein aggregation into amyloid structure (Bieschke, 2013). Different proteins and peptides form amyloid aggregates in case of each disease, but all aggregates share similar physicochemical and structural properties (Chiti & Dobson, 2006). Structurally similar aggregates, referred to as amyloid-like fibrils (Nelson & Eisenberg, 2006), can be formed from both disease-related and unrelated proteins and peptides in vitro. This led to the idea that amyloid-like structure may be a generic property of polypeptide chains (Chiti & Dobson, 2006). Given structural similarities of different amyloid and amyloid-like fibrils, there is a possibility that generic inhibitors of amyloid fibril formation may exist.
A number of small molecules were reported as inhibitors of amyloid-like fibril formation (Doig & Derreumaux, 2015; Seneci, 2015), some of them reached different phases of clinical trials, but none is approved as a drug yet (Mangialasche et al., 2010; Seneci, 2015). One of the best-known inhibitors of protein amyloid fibrillation is epi-gallocatechine-3-gallate (EGCG). There are reports, suggesting its inhibitory effect on the fibril formation of amyloid beta (Abeta) peptide and alpha-synuclein (Ehrnhoefer et al., 2008; Roberts & Shorter, 2008; Bieschke et al., 2010), huntingtin (Ehrnhoefer et al., 2006), mammalian and yeast prions (Rambold et al., 2008; Roberts et al., 2009), kappa-casein (Hudson et al., 2009a), transthyretin (Ferreira et al., 2009; Ferreira, Saraiva & Almeida, 2011), islet amyloid polypeptide (Meng et al., 2010), Plasmodium falciparum merozoite surface protein 2 (Chandrashekaran et al., 2010; Chandrashekaran et al., 2011), human insulin (Wang, Dong & Sun, 2012), hen egg white lysozyme (Ghosh, Pandey & Dasgupta, 2013), tau protein (Wobst et al., 2015), and human parathyroid hormone (Gopalswamy et al., 2015). The large number and variety of targets suggested that EGCG is a genuine generic inhibitor of amyloid fibril formation. Resveratrol is another compound inhibiting amyloid-like fibril formation of several proteins, including Abeta (Feng et al., 2009; Ladiwala et al., 2010), alpha-synuclein (Herva et al., 2014), and islet amyloid polypeptide (Mishra et al., 2009). A number of different flavone derivatives, including morin, quercetin, fisetin and luteolin were reported as inhibitors of Abeta fibrillation (Ono et al., 2003; Akaishi et al., 2008; Ushikubo et al., 2012). Luteolin, quercetin and fisetin can inhibit transthyretin aggregation (Trivella et al., 2012), and luteolin also inhibits fibrillation of insulin (Malisauskas et al., 2015). There is a report on islet amyloid polypeptide inhibition by morin (Noor, Cao & Raleigh, 2012). Our interest in flavones as inhibitors of amyloid-like fibril formation was especially raised by the study of Akaishi et al. (2008), which suggested that inhibitory effect of flavone derivatives is dependent on the number and positions of hydroxyl group around the flavone backbone and a subsequent work of Ushikubo et al. (2012), which designed a new flavone-derived inhibitor of Abeta aggregation.
One of the major problems in the detection of anti-amyloid compounds is ambiguity of the methods used for screening. A significant portion of the studies referenced relied only on changes in maximal ThT fluorescence intensity to establish inhibition of fibril formation (Ono et al., 2003; Akaishi et al., 2008; Ushikubo et al., 2012), sometimes leading to controversial results. For example Ono et al. (2003) claimed kaempferol as an inhibitor, while Akaishi et al. (2008) showed it to enhance Abeta fibril formation. Other studies have described how ThT fluorescence intensity can be affected by different compounds (Foderà et al., 2008; Hudson et al., 2009b; Noormägi et al., 2012).
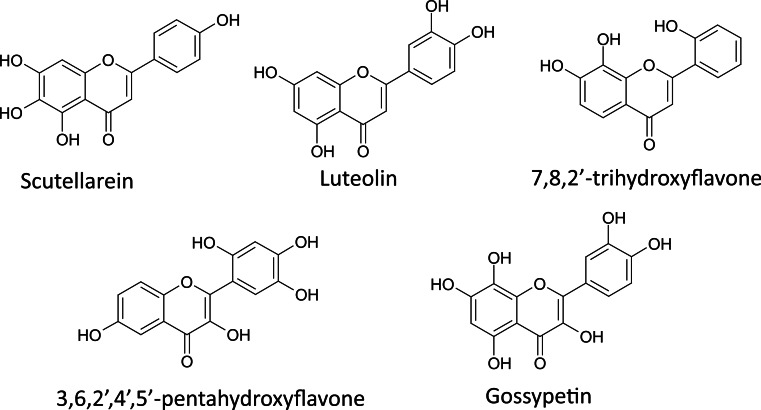
Recently, we demonstrated the ability to avoid false-positives in ThT fluorescence assay-based screening by comparing halftimes of aggregation (t50) rather than fluorescence intensities (Malisauskas et al., 2015). Screening of 265 flavone derivatives based on halftimes of aggregation let us to identify 5 outstanding inhibitors (Fig. 1) of insulin amyloid-like fibril formation (Malisauskas et al., 2015). In order to further test our screening method and identify a possible generic inhibitor of amyloid-like fibril formation among these flavone derivatives, we checked their effect on the aggregation of alpha-synuclein and Abeta.
Figure 1. The best flavone inhibitors of insulin amyloid-like fibril formation.
Materials and Methods
Flavones were purchased from Indofine Chemical Company and EGCG was purchased from Sigma. Flavones and EGCG were dissolved at a concentration of 20 mM in dimethylsulfoxide (DMSO) and stored in the dark at room temperature for up to two weeks.
Production of alpha-synuclein
E. coli BL-21(DE3) (Invitrogen) was used as the host strain for the over-expression of alpha-synuclein. For this purpose, cells harbouring a plasmid pRK172 were grown in a standard NB medium supplemented with 50 µg/mL ampicillin. 200 mL of medium was inoculated with 1 mL of the overnight culture and incubated at 30 °C until an OD600 of 0.7–0.8 was reached. Protein expression was then induced by adding IPTG to a final concentration of 0.2 mM, and the incubation was continued for additional 18 h. The cells were harvested by centrifugation for 30 min at 4,000 g (4 °C), resuspended in 20 mM Tris-HCl buffer (pH 8.0), containing 0.5 M NaCl, 1 mM PMSF and 1 mM EDTA and disrupted by sonication at 22 kHz for 3 min., using 50% amplitude. To remove cellular debris, the cell lysate was centrifuged at 10,000 g for 20 min at 4 °C. After centrifugation, cellular extract was subjected to a 20 min. heat treatment using a water bath at 100 °C. Cell extract with aggregated proteins was immediately centrifuged at 10,000 g for 30 min. at 4 °C. The resulting clear supernatant was dialysed at 4 °C for 18 h against 20 mM Tris-HCl buffer (pH 8.0), containing 1 mM EDTA and 1 mM DTT (buffer A). The desalted sample was applied at a flow rate of 1 mL/min onto a 5 mL HiTrap ANX HP column (GE Healthcare, Little Chalfont, UK), previously equilibrated with buffer A. After washing with 5 column volumes of buffer A, the recombinant protein was eluted using a linear gradient of 0–1 M NaCl in buffer A. The eluted from the column fractions were checked by SDS electrophoresis, pooled and dialyzed overnight against buffer A. The dialyzed protein solution was applied at a flow rate of 0.5 mL/min onto second ion exchange 1 mL HiTrap Q XL column (GE Healthcare) equilibrated with buffer A. After a 5 column volume wash with buffer A, alpha-synuclein was eluted over a linear gradient of 0–1 M NaCl in buffer A. The major peak eluted from the column was checked by electrophoresis, pooled and dialyzed overnight against 5 mM ammonium carbonate buffer (pH 7.6). Desalted protein samples were flash-frozen, lyophilized and stored at −20 °C until use. The homogeneity of protein was verified by SDS-PAGE. Protein concentration was determined using the Lowry method with bovine serum albumin as the standard.
Production of abeta
The expression vector for Abeta42 in E. coli was described previously (Walsh et al., 2009; Vignaud et al., 2013). The recombinant Abeta peptide was expressed in E. coli BL-21Star™ (DE3) (Invitrogen, Carlsbad, California, USA) and purified similarly to a previously described method (Walsh et al., 2009; Hellstrand et al., 2010; Vignaud et al., 2013). The expression vector for Abeta42 peptide was transformed into Ca2+-competent E. coli cells by heat shock and spread on LB agar plates containing ampicillin (100 µg/mL). Single colonies were used to inoculate 100 mL overnight cultures in LB medium with ampicillin (100 µg/mL). The next morning 1 mL of overnight culture was transferred to 400 mL of auto-inductive ZYM-5052 medium (Studier, 2005) containing ampicillin (100 µg/mL) and grown for 24 h. The cell suspension was centrifuged at 5,000 g and 4 °C for 15 min. The cell pellet was frozen. The frozen cell pellet from 3.6 L culture was thawed, homogenized with Potter–Elvehjem homogenizer in a total of 100 mL buffer B (10 mM Tris/HCl pH 8.0, 1 mM EDTA) and sonicated for 10 min on ice (30s/30s horn, 50% duty cycle). Cell pellet was centrifuged at 18,000 g and 4 °C for 15 min. The supernatant was removed, and the pellet was resuspended twice in 100 mL of buffer B, homogenized and centrifuged as above. The third supernatant was removed and the pellet was resuspended in 50 mL of buffer C (8 M urea, 10 mM Tris/HCl pH 8.0, 1 mM EDTA), homogenized and centrifuged as above, resulting in clear solution. The urea-solubilized inclusion bodies (50 mL) were diluted with 150 mL of buffer C, added to 50 mL DEAE-sepharose equilibrated in buffer C, and gently agitated (80 rpm) for 30 min at 4 °C. The slurry was then applied to a Büchner funnel with Fisherbrand glass microfibers paper on a vacuum glass bottle. Later, the resin was washed with 50 mL of buffer C and then with 50 mL of buffer C with 25 mM NaCl followed by four aliquots of buffer C with 125 mM NaCl. Each aliquot was incubated with the resin for 5 min before collection under vacuum. The presence of the peptide in aliquots was tested using Tricine SDS-PAGE (Schägger, 2006). Combined aliquots (200 mL) were centrifuged through 30 kDa molecular weight cutoff (MWCO) filter, and finally concentrated approximately twenty fold using a 3 kDa MWCO filter. The purified peptide was frozen in 1 mL aliquots. Aliquots of purified peptide were thawed, diluted with 3 mL of buffer D (8 M GuHCl, 50 mM Tris/HCl pH 8.0) and concentrated to the final volume of ∼300 µL using 3 kDa MWCO concentrator. Concentrated samples were loaded on a Tricorn 10/300 column (packed with Superdex 75 gelfiltration resin), and eluted at 1 mL/min using buffer E (20 mM sodium phosphate pH 8.0, 200 µM EDTA). Collected fractions (2 mL) were diluted with 6 mL of buffer D, concentrated, and the chromatography was repeated. After repetitive purification the Abeta monomer peak was collected on ice. The amount of purified Abeta was calculated by integration of the chromatographic UV absorbance peak, using extinction coefficient E280 = 1,490 M−1 cm−1.
Aggregation of abeta
Freshly purified (within 30 min after gelfiltration) monomeric Abeta42 solution was supplemented with 50 µM ThT and diluted using buffer E containing 50 µM ThT to reach different concentrations (1–6 µM). For the inhibition experiments 216, 36, 6, 1, and 0.167 µM solutions of flavones in buffer D, containing 2% DMSO and 50 µM ThT were prepared. Monomeric 6 µM Abeta42 solution was mixed with these flavone derivative solutions (or with buffer E containing 2% DMSO as a control) in a 1:1 ratio. Each sample was divided into three 100 µL aliquots into wells of a 96 well non-binding half-area plate (Corning NBS™). Kinetics of aggregation was followed at 37 °C temperature and constant shaking (960 rpm) using Synergy H4 Hybrid Multi-Mode (Biotek, Winooski, Vermont, USA) microplate reader. The intensity of ThT fluorescence was measured through the bottom of the plate every 3 min using 440 nm excitation and 482 nm emission.
Aggregation of alpha-synuclein
Alpha-synuclein was dissolved in 30 mM Tris-HCl buffer (pH 7.5), containing 0.05% sodium azide and 50 µM ThT. For the study of the concentration dependence of fibrillation, a range of alpha-synuclein concentrations between 1 and 5 mg/mL (69–345 µM) was used, and for inhibition studies 150 µM final concentration was used. For the inhibition experiments 450, 375, 300, 225, 150, and 75 µM solutions of flavones in 30 mM Tris-HCl buffer (pH 7.5), containing 0.05% sodium azide, 2.25% DMSO, and 50 µM ThT were prepared. The 300 µM alpha-synuclein solution was mixed with flavones in a 1:1 ratio. Each sample was divided into three 160 µL aliquots into wells of a 96 well plate (Fisherbrand, Waltham, Massachusetts, USA) and one ∼3 mm glass bead was added into each well. Kinetics of aggregation was followed at 60 °C temperature and constant shaking (960 rpm) using Synergy H4 Hybrid Multi-Mode microplate reader. The intensity of ThT fluorescence was measured through the bottom of the plate every 2 min using 440 nm excitation and 482 nm emission.
Extraction of relative t50 and Imax values
Maximal fluorescence intensities and halftimes of aggregation were obtained by fitting experimental data using following sigmoidal equation:
where I is the intensity of fluorescence, x is time, t50 is the time to 50% of maximal fluorescence intensity, Imin and Imax are the minimal and maximal intensity of ThT fluorescence and n is the Hill coefficient. Fitting was performed using Origin 8.1 software. Extracted t50 and Imax values in the presence of flavone derivatives were divided by the values for the control samples to obtain relative t50 and Imax. Average values and errors were calculated using three different batch preparations of flavones and three samples within each batch (a total of 9 repeats per flavone derivative).
Congo Red spectroscopic assay
The assay was performed as described previously (Nilsson, 2004). Briefly, 7 mg/mL Congo Red stock solution was prepared in 30 mM Tris buffer, pH 7.5, and filtered through 0.22 µm syringe filter and stored at room temperature for up to a week. Alpha-synuclein samples were taken at different times of aggregation. 50 µL of alpha-synuclein sample was mixed with 50 µL of 70 µg/mL Congo Red (freshly prepared from stock solution) and incubated for 30 min at room temperature in 96 well non-binding half-area plate (Corning NBSTM). Spectra were recorded using Synergy H4 Hybrid Multi-Mode (Biotek) microplate reader. In case of Abeta, 35 µg/mL of Congo Red were added to the 15 µM peptide samples at the beginning of the reaction. 100 µL aliquots were transferred into wells of a 96 well non-binding half-area plate (Corning NBS; Corning Inc., Corning, New York, USA). The plate was incubated at 37 °C temperature, and spectra were recorded at different time points using Synergy H4 Hybrid Multi-Mode (Biotek) microplate reader. To get differential spectra, the corresponding spectrum at zero time point was mathematically subtracted from the spectra at later time points.
Atomic force microscopy (AFM)
For atomic force microscopy experiments, 20 µL of the sample and 10 µL of 1 M HCl (protein fibrils in neutral/basic buffer have net negative charge and poorly adsorb on the mica) were mixed on freshly cleaved mica and left to adsorb for 1 min, the sample was rinsed with several mL of water and dried gently using airflow. AFM images were recorded in the Tapping-in-Air mode at a drive frequency of approximately 300 kHz, using a Dimension Icon (Bruker, Santa Barbara, California, USA) scanning probe microscope system. Aluminium-coated silicon tips (RTESPA-300; Bruker, Billerica, Massachusetts, USA) from Bruker were used as a probe.
Results
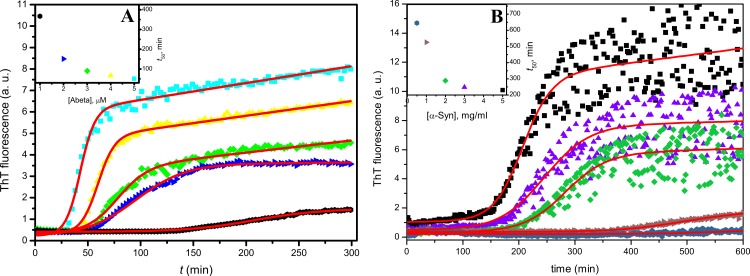
The first step of our study was the optimization of kinetic assays of Abeta and alpha-synuclein. As the best flavone inhibitors increased t50 of insulin aggregation up to 24 times (Malisauskas et al., 2015), we tried to find conditions, where t50 values of Abeta and alpha-synuclein aggregation would not exceed several hours. A way to get fast and highly reproducible kinetics of Abeta42 aggregation was recently described by the Linse group (Hellstrand et al., 2010). We were able to use it with similar results (Fig. 2A). Alpha-synuclein aggregates slower than Abeta; however, the rate of aggregation can be increased using agitation, beads, and higher temperature (Buell et al., 2014). The dependence of t50 values on the concentration of alpha-synuclein is shown in Fig. 2B. For further experiments, we chose the optimal concentrations of peptides (3 µM for Abeta and 150 µM for alpha-synuclein).
Figure 2. Concentration dependence of Abeta (A), and alpha-synuclein (B) aggregation kinetics.
Raw data at different peptide concentrations is represented by scatter plots of different colors; fitting is represented by red curves. Inserts show concentration dependences of t50 values and also serves as color-code legends.
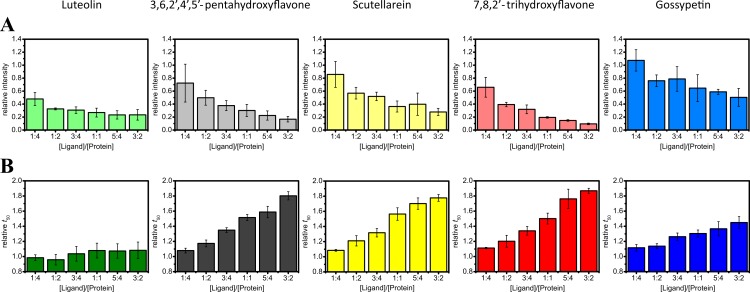
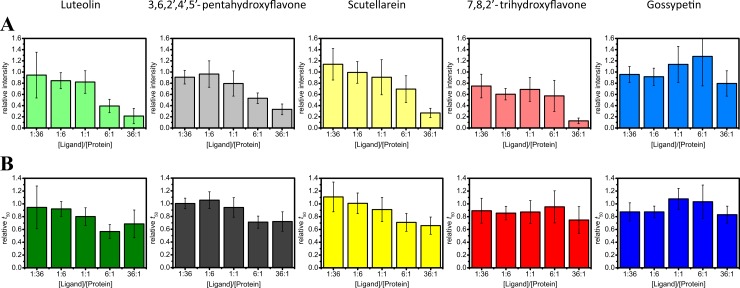
The impact of flavones on ThT fluorescence intensity and t50 values of alpha-synuclein aggregation is summarized in the Fig. 3. The effect of luteolin differs from other flavones. ThT fluorescence is strongly affected even by lowest luteolin concentrations, but relative t50 values stay close to 1. Other flavones quench ThT fluorescence and increase the time of alpha-synuclein aggregation in a concentration-dependent manner. The inhibitory effect of gossypetin looks about twice lower than scutellarein, 7,8,2′-trihydroxyflavone or 3,6,2′,4′,5′-pentahydroxyflavone.
Figure 3. Effect of flavones on the aggregation of alpha-synuclein.
Each flavone derivative is represented by different color. Relative ThT fluorescence intensities are shown in light colors (A) and relative halftimes of aggregation in dark colors (B).
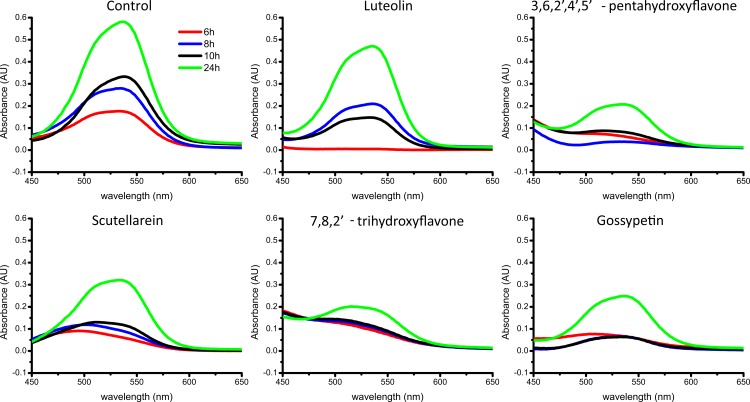
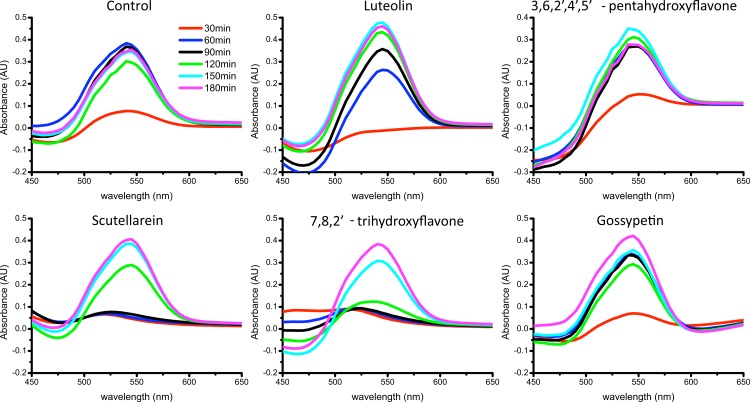
The complementary analysis using Congo Red spectroscopic assay gave similar results (Fig. 4). Spectral maxima at ∼540 nm show the fastest and highest increases in the control sample and in the presence of luteolin. In the presence of the rest of the flavones, there is just a minor rise within 10 h and smaller final absorbance after 24 h of incubation. The data suggest all tested flavones except luteolin inhibit aggregation of alpha-synuclein.
Figure 4. Aggregation of alpha-synuclein in the presence of flavones followed by Congo Red differential spectra.
The ligand/protein ratio was 3:2.
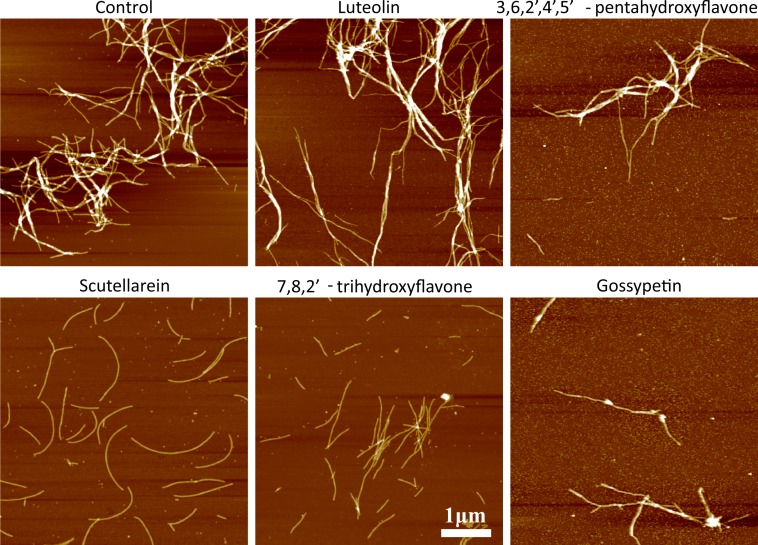
Microscopy data revealed that amyloid-like fibrils still can be formed in the presence of inhibitors (Fig. 5); however, some differences are worth mentionining. In the absence of flavones and in the presence of luteolin big fibril clumps can be found. In the presence of scutellarein and 7,8,2′-trihydroxyflavone, images contain mostly separate 4 nm diameter fibrils, while in the presence of 3,6, 2′,4′,5′-pentahydroxyflavone and gossypetin some fibril clumps and a number of 2–4 nm oligomers can be found. It suggests that all tested flavones but luteolin change not only time of aggregation but also the amount and the nature of final aggregates.
Figure 5. AFM images of alpha synuclein aggregates.
The ligand/protein ratio was 3:2.
Equimolar amounts of flavones had almost no influence on both ThT fluorescence intensity and t50 values of Abeta aggregation. In Fig. 6 we show the data, obtained using up to 36 times higher concentrations of compounds. Both fluorescence intensity and t50 values of the aggregation in the presence of gossypetin show only minor decreases when compared to the control. In the presence of the highest used concentrations of other flavones, fluorescence intensity strongly decreases, but the time of aggregation is not increased. Moreover, the aggregation looks a little faster.
Figure 6. Effect of flavones on the aggregation of Abeta.
Each flavone derivative is represented by different color. Relative ThT fluorescence intensities are shown in light colors (A) and relative halftimes of aggregation in dark colors (B).
Complementary analysis using Congo Red spectroscopic assay showed some differences (Fig. 7). The spectral maxima at ∼540 nm grow fast in the control sample and in the presence of luteolin, 3,6, 2′,4′,5′-pentahydroxyflavone and gossypetin. In samples with scuttelarein and 7,8,2′-trihydroxyflavone the raise is stalled for the first 90 min; however, it reached similar maxima as in the control sample within 3 h. It can be interpreted as an inhibition of the initial steps of fibrillation; however the effect is not major.
Figure 7. Aggregation of Abeta in the presence of flavones followed by Congo Red differential spectra.
Abeta concentration was 15 µ M. The ligand/protein ratio was 7:1.
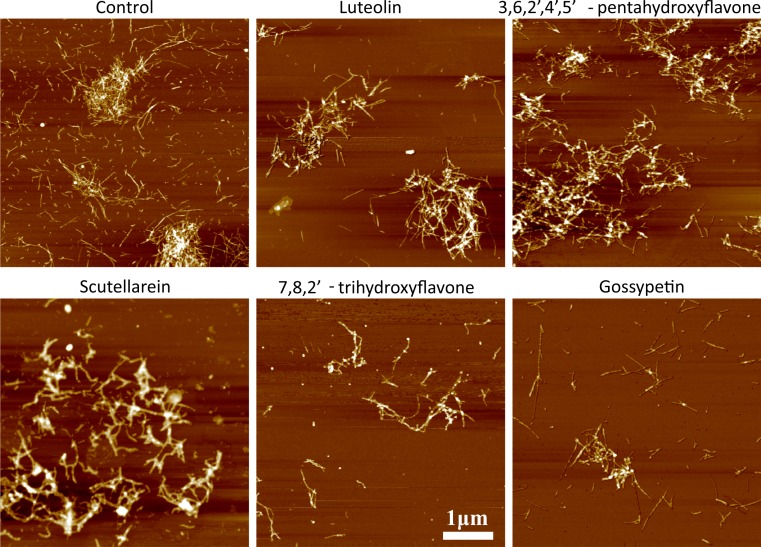
No major differences were found in the AFM images of Abeta fibrils formed in the presence of different flavone derivatives (Fig. 8). The diameter of individual fibrils in all cases is in the range of 4–10 nm and the majority of fibrils are in larger clumps.
Figure 8. AFM images of Abeta aggregates. Abeta concentration was 15 µM.
The ligand/protein ratio was 7:1.
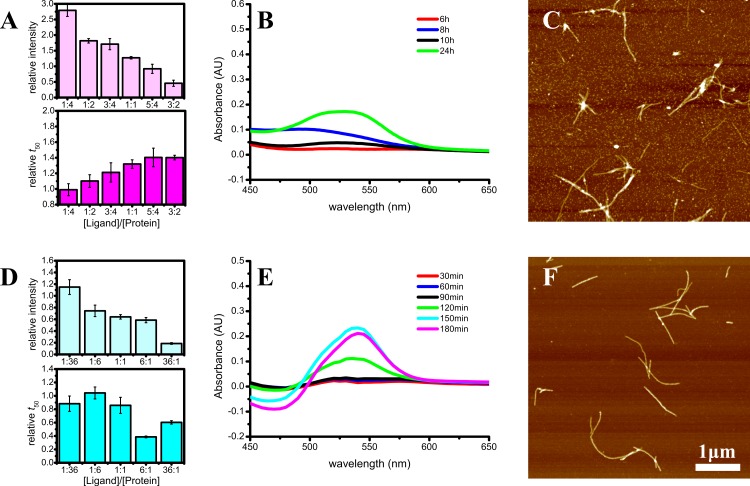
As a positive control of inhibition, we used EGCG (Fig. 9). In case of alpha-synuclein it works similar to most of the tested flavones. ThT fluorescence intensity is decreased and time of aggregation is increased with higher EGCG concentrations (Fig. 9A). Congo Red differential spectral maxima rise slowly and final absorbance values are low (Fig. 9B). Finally, a number of oligomers were detected together with the final fibrils (Fig. 9C). The only difference is higher ThT fluorescence intensities at low EGCG concentrations. In the case of Abeta, ThT intensity decrease and fluctuations of aggregation time are also comparable to the data seen in the presence of flavones (Fig. 9D). The Congo Red spectral maximum starts to rise after 2 h, similarly to the cases of scuttelarein and 7,8,2′-trihydroxyflavone (Fig. 9E). The diameter of Abeta fibrils formed in the presence of EGCG is also similar to the control sample; however, the amount of fibrils found on the mica is lower.
Figure 9. Aggregation of alpha-synuclein (A–C) and Abeta (D–F) in presence of EGCG.
Illustrated by relative ThT intensity and t50 (A and D), Congo Red differential spectra (B and E) and AFM images of final aggregates (C and F). Abeta concentration used for Congo Red and AFM studies was 15 µM. The EGCG/Abeta ratio was 7:1, and EGCG/alpha-synuclein ratio was 3:2.
Discussion
We began the current study with hopes of finding a useful, generic inhibitor of amyloid fibril formation. With the variety of literature reporting flavones as effective inhibitors, we previously carried out an extensive screening of 265 commercially available flavone derivatives. The study revealed a method for eliminating large numbers of false positives for fibril inhibition while still using a simple method—ThT fluorescence. By measuring the time for 50% of maximal fibril growth rather than absolute ThT fluorescence, we identified only five flavones that appreciably inhibited insulin fibril formation (Malisauskas et al., 2015). In extending the study to look at the effects of these five flavones on Abeta and alpha-synuclein, we found moderate to no inhibition of fibril formation for either of these proteins. If there is an effective, generic inhibitor of fibril formation to be found among flavone derivatives, it is not among the 265 commercially available compounds.
Surprisingly, an expected positive control (EGCG) worked similar to some flavones. Our results show that EGCG does not inhibit Abeta fibrillation, leading to the conclusion that it is not the universal inhibitor implied by the collection of previous reports. In fact, our ThT data on aggregation of alpha-synuclein and Abeta in the presence of EGCG are comparable to the previously published (Ehrnhoefer et al., 2008) and the main controversy comes from the microscopy data. Ehrnhoefer et al. (2008) found just oligomers and no fibrils in alpha-synuclein and Abeta samples in the presence of EGCG; however, we can see mostly fibrils in Abeta samples (Fig. 9F) and fibrils together with oligomers in alpha-synuclein samples (Fig. 9C). Having in mind low stability of EGCG at neutral pH (Zhu et al., 1997) and the fact that EGCG effect on amyloid is dependent on auto-oxidation of EGCG (Palhano et al., 2013), slightly different conditions for our experiments (compared to Ehrnhoefer et al.) may be the reason of controversial data. If this is true, then even EGCG cannot be called universal inhibitor of amyloid aggregation, as inhibition may be dependent on the environmental conditions.
Failure appears to be a common state of affairs when discussing drug development for Alzheimer’s disease (Becker, Greig & Giacobini, 2008; Rosenblum, 2014; Schneider et al., 2014). While failures in clinical trials involve much greater complexity than the (relatively) simple screening of small molecule amyloid inhibitors, many candidate molecules for drug trials come from high-throughput screening, so the method of screening is directly responsible for the selection of the right targets. As noted in the introduction and confirmed in our current study, false positive results for amyloid inhibition can lead to extensive resources expended characterizing the effects of molecules that will ultimately be of no benefit in treating disease. Had we relied solely on maximum ThT fluorescence intensity to determine inhibition, four out of the five flavones studied would be indicated as inhibitors of Abeta aggregation. In fact, luteolin was already mentioned as an inhibitor previously (Akaishi et al., 2008). Of course, there is always thorough testing between initial screenings of drug candidates and clinical trials, but our simple expedient of measuring a kinetic feature of fibril aggregation has proven sufficient to eliminate many false positives with Abeta and alpha-synuclein fibrillation, in addition to our previous observations for insulin fibrillation.
Measuring the time to half maximal ThT fluorescence has proved so effective in the case of 265 flavones that we reduced a large false positive rate for inhibition to the point where we can demonstrate that there is no universal amyloid inhibitor among these candidate compounds. The more labor intensive, additional studies of measuring Congo Red absorbance spectra and AFM added some interesting information, but did not improve on our ability to quickly reduce a large set of candidate inhibitors to a small set of more promising candidates. However, care is clearly required before drawing sweeping conclusions from these simple results as illustrated by the discrepancies between our observations and those published previously regarding EGCG as an inhibitor of Abeta and alpha-synuclein fibrillation (Ehrnhoefer et al., 2008; Bieschke et al., 2010). These controversial data emphasize the ambiguity of methods applied for the detection of anti-amyloid compounds and suggests that differences in enviromental conditions must be carefully examined in seeking a full understanding of inhibitor effects.
Supplemental Information
Raw data
Raw data
Acknowledgments
The expression plasmid (pRK 172) harbouring gene for human alpha-synuclein was kindly provided by Dr. LA Morozova-Roche and Dr. M Malisauskas. The expression plasmid with Abeta42 gene was kindly provided by Dr. C Cullin. The authors thank Dr. M Jankunec for the help with AFM.
Funding Statement
This research was funded by the European Social Fund under the Global Grant Measure, project number VP1-3.1-ŠMM-07-K-02-020. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Tomas Šneideris and Lina Baranauskienė conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, reviewed drafts of the paper.
Jonathan G. Cannon wrote the paper, reviewed drafts of the paper.
Rasa Rutkienė and Rolandas Meškys contributed reagents/materials/analysis tools, reviewed drafts of the paper.
Vytautas Smirnovas conceived and designed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
References
- Akaishi et al. (2008).Akaishi T, Morimoto T, Shibao M, Watanabe S, Sakai-Kato K, Utsunomiya-Tate N, Abe K. Structural requirements for the flavonoid fisetin in inhibiting fibril formation of amyloid beta protein. Neuroscience Letters. 2008;444:280–285. doi: 10.1016/j.neulet.2008.08.052. [DOI] [PubMed] [Google Scholar]
- Becker, Greig & Giacobini (2008).Becker RE, Greig NH, Giacobini E. Why do so many drugs for Alzheimer’s disease fail in development? Time for new methods and new practices? Journal of Alzheimer’s Disease. 2008;15:303–325. doi: 10.3233/jad-2008-15213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieschke (2013).Bieschke J. Natural compounds may open new routes to treatment of amyloid diseases. Neurotherapeutics. 2013;10:429–439. doi: 10.1007/s13311-013-0192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieschke et al. (2010).Bieschke J, Russ J, Friedrich RP, Ehrnhoefer DE, Wobst H, Neugebauer K, Wanker EE. EGCG remodels mature alpha-synuclein and amyloid-beta fibrils and reduces cellular toxicity. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:7710–7715. doi: 10.1073/pnas.0910723107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buell et al. (2014).Buell AK, Galvagnion C, Gaspar R, Sparr E, Vendruscolo M, Knowles TPJ, Linse S, Dobson CM. Solution conditions determine the relative importance of nucleation and growth processes in α-synuclein aggregation. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:7671–7676. doi: 10.1073/pnas.1315346111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekaran et al. (2010).Chandrashekaran IR, Adda CG, MacRaild CA, Anders RF, Norton RS. Inhibition by flavonoids of amyloid-like fibril formation by Plasmodium falciparum merozoite surface protein 2. Biochemistry. 2010;49:5899–5908. doi: 10.1021/bi902197x. [DOI] [PubMed] [Google Scholar]
- Chandrashekaran et al. (2011).Chandrashekaran IR, Adda CG, MacRaild CA, Anders RF, Norton RS. EGCG disaggregates amyloid-like fibrils formed by Plasmodium falciparum merozoite surface protein 2. Archives of Biochemistry and Biophysics. 2011;513:153–157. doi: 10.1016/j.abb.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiti & Dobson (2006).Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annual Review of Biochemistry. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- Doig & Derreumaux (2015).Doig AJ, Derreumaux P. Inhibition of protein aggregation and amyloid formation by small molecules. Current Opinion in Structural Biology. 2015;30:50–56. doi: 10.1016/j.sbi.2014.12.004. [DOI] [PubMed] [Google Scholar]
- Ehrnhoefer et al. (2006).Ehrnhoefer DE, Duennwald M, Markovic P, Wacker JL, Engemann S, Roark M, Legleiter J, Marsh JL, Thompson LM, Lindquist S, Muchowski PJ, Wanker EE. Green tea (-)-epigallocatechin-gallate modulates early events in huntingtin misfolding and reduces toxicity in Huntington’s disease models. Human Molecular Genetics. 2006;15:2743–2751. doi: 10.1093/hmg/ddl210. [DOI] [PubMed] [Google Scholar]
- Ehrnhoefer et al. (2008).Ehrnhoefer DE, Bieschke J, Boeddrich A, Herbst M, Masino L, Lurz R, Engemann S, Pastore A, Wanker EE. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nature Structural & Molecular Biology. 2008;15:558–566. doi: 10.1038/nsmb.1437. [DOI] [PubMed] [Google Scholar]
- Feng et al. (2009).Feng Y, Wang XP, Yang SG, Wang YJ, Zhang X, Du XT, Sun XX, Zhao M, Huang L, Liu RT. Resveratrol inhibits beta-amyloid oligomeric cytotoxicity but does not prevent oligomer formation. Neuro Toxicology. 2009;30:986–995. doi: 10.1016/j.neuro.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Ferreira et al. (2009).Ferreira N, Cardoso I, Domingues MR, Vitorino R, Bastos M, Bai G, Saraiva MJ, Almeida MR. Binding of epigallocatechin-3-gallate to transthyretin modulates its amyloidogenicity. FEBS Letters. 2009;583:3569–3576. doi: 10.1016/j.febslet.2009.10.062. [DOI] [PubMed] [Google Scholar]
- Ferreira, Saraiva & Almeida (2011).Ferreira N, Saraiva MJ, Almeida MR. Natural polyphenols inhibit different steps of the process of transthyretin (TTR) amyloid fibril formation. FEBS Letters. 2011;585:2424–2430. doi: 10.1016/j.febslet.2011.06.030. [DOI] [PubMed] [Google Scholar]
- Foderà et al. (2008).Foderà V, Groenning M, Vetri V, Librizzi F, Spagnolo S, Cornett C, Olsen L, Van De Weert M, Leone M. Thioflavin T hydroxylation at basic pH and its effect on amyloid fibril detection. Journal of Physical Chemistry B. 2008;112:15174–15181. doi: 10.1021/jp805560c. [DOI] [PubMed] [Google Scholar]
- Ghosh, Pandey & Dasgupta (2013).Ghosh S, Pandey NK, Dasgupta S. (-)-Epicatechin gallate prevents alkali-salt mediated fibrillogenesis of hen egg white lysozyme. International Journal of Biological Macromolecules. 2013;54:90–98. doi: 10.1016/j.ijbiomac.2012.11.031. [DOI] [PubMed] [Google Scholar]
- Gopalswamy et al. (2015).Gopalswamy M, Kumar A, Adler J, Baumann M, Henze M, Kumar ST, Fändrich M, Scheidt HA, Huster D, Balbach J. Structural characterization of amyloid fibrils from the human parathyroid hormone. Biochimica et Biophysica Acta (BBA)—Proteins and Proteomics. 2015;1854:249–257. doi: 10.1016/j.bbapap.2014.12.020. [DOI] [PubMed] [Google Scholar]
- Hellstrand et al. (2010).Hellstrand E, Boland B, Walsh DM, Linse S. Amyloid beta-protein aggregation produces highly reproducible kinetic data and occurs by a two-phase process. ACS Chemical Neuroscience. 2010;1:13–18. doi: 10.1021/cn900015v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herva et al. (2014).Herva ME, Zibaee S, Fraser G, Barker RA, Goedert M, Spillantini MG. Anti-amyloid compounds inhibit alpha-synuclein aggregation induced by protein misfolding cyclic amplification (PMCA) Journal of Biological Chemistry. 2014;289:11897–11905. doi: 10.1074/jbc.M113.542340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson et al. (2009a).Hudson SA, Ecroyd H, Dehle FC, Musgrave IF, Carver JA. (-)-Epigallocatechin-3-Gallate (EGCG) maintains κ-casein in its pre-fibrillar state without redirecting its aggregation pathway. Journal of Molecular Biology. 2009a;392:689–700. doi: 10.1016/j.jmb.2009.07.031. [DOI] [PubMed] [Google Scholar]
- Hudson et al. (2009b).Hudson SA, Ecroyd H, Kee TW, Carver JA. The thioflavin T fluorescence assay for amyloid fibril detection can be biased by the presence of exogenous compounds. FEBS Journal. 2009b;276:5960–5972. doi: 10.1111/j.1742-4658.2009.07307.x. [DOI] [PubMed] [Google Scholar]
- Ladiwala et al. (2010).Ladiwala ARA, Lin JC, Bale SS, Marcelino-Cruz AM, Bhattacharya M, Dordick JS, Tessier PM. Resveratrol selectively remodels soluble oligomers and fibrils of amyloid Abeta into off-pathway conformers. Journal of Biological Chemistry. 2010;285:24228–24237. doi: 10.1074/jbc.M110.133108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malisauskas et al. (2015).Malisauskas R, Botyriute A, Cannon JG, Smirnovas V. Flavone derivatives as inhibitors of insulin amyloid-like fibril formation. PLoS ONE. 2015;10:e1271. doi: 10.1371/journal.pone.0121231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangialasche et al. (2010).Mangialasche F, Solomon A, Winblad B, Mecocci P, Kivipelto M. Alzheimer’s disease: clinical trials and drug development. The Lancet Neurology. 2010;9:702–716. doi: 10.1016/S1474-4422(10)70119-8. [DOI] [PubMed] [Google Scholar]
- Meng et al. (2010).Meng F, Abedini A, Plesner A, Verchere CB, Raleigh DP. The flavanol (-)-epigallocatechin 3-gallate inhibits amyloid formation by islet amyloid polypeptide, disaggregates amyloid fibrils, and protects cultured cells against IAPP-induced toxicity. Biochemistry. 2010;49:8127–8133. doi: 10.1021/bi100939a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra et al. (2009).Mishra R, Sellin D, Radovan D, Gohlke A, Winter R. Inhibiting islet amyloid polypeptide fibril formation by the red wine compound resveratrol. ChemBioChem. 2009;10:445–449. doi: 10.1002/cbic.200800762. [DOI] [PubMed] [Google Scholar]
- Nelson & Eisenberg (2006).Nelson R, Eisenberg D. Structural models of amyloid-like fibrils. Advances in Protein Chemistry. 2006;73:235–282. doi: 10.1016/S0065-3233(06)73008-X. [DOI] [PubMed] [Google Scholar]
- Nilsson (2004).Nilsson MR. Techniques to study amyloid fibril formation in vitro. Methods. 2004;34:151–160. doi: 10.1016/j.ymeth.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Noor, Cao & Raleigh (2012).Noor H, Cao P, Raleigh DP. Morin hydrate inhibits amyloid formation by islet amyloid polypeptide and disaggregates amyloid fibers. Protein Science. 2012;21:373–382. doi: 10.1002/pro.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noormägi et al. (2012).Noormägi A, Primar K, Tõugu V, Palumaa P. Interference of low-molecular substances with the thioflavin-T fluorescence assay of amyloid fibrils. Journal of Peptide Science. 2012;18:59–64. doi: 10.1002/psc.1416. [DOI] [PubMed] [Google Scholar]
- Ono et al. (2003).Ono K, Yoshiike Y, Takashima A, Hasegawa K, Naiki H, Yamada M. Potent anti-amyloidogenic and fibril-destabilizing effects of polyphenols in vitro: implications for the prevention and therapeutics of Alzheimer’s disease. Journal of Neurochemistry. 2003;87:172–181. doi: 10.1046/j.1471-4159.2003.01976.x. [DOI] [PubMed] [Google Scholar]
- Palhano et al. (2013).Palhano FL, Lee J, Grimster NP, Kelly JW. Toward the molecular mechanism(s) by which EGCG treatment remodels mature amyloid fibrils. Journal of the American Chemical Society. 2013;135:7503–7510. doi: 10.1021/ja3115696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambold et al. (2008).Rambold AS, Miesbauer M, Olschewski D, Seidel R, Riemer C, Smale L, Brumm L, Levy M, Gazit E, Oesterhelt D, Baier M, Becker CFW, Engelhard M, Winklhofer KF, Tatzelt J. Green tea extracts interfere with the stress-protective activity of PrPC and the formation of PrPSc. Journal of Neurochemistry. 2008;107:218–229. doi: 10.1111/j.1471-4159.2008.05611.x. [DOI] [PubMed] [Google Scholar]
- Roberts et al. (2009).Roberts BE, Duennwald ML, Wang H, Chung C, Lopreiato NP, Sweeny E a, Knight MN, Shorter J. A synergistic small-molecule combination directly eradicates diverse prion strain structures. Nature Chemical Biology. 2009;5:936–946. doi: 10.1038/nchembio.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts & Shorter (2008).Roberts BE, Shorter J. Escaping amyloid fate. Nature Structural & Molecular Biology. 2008;15:544–546. doi: 10.1038/nsmb0608-544. [DOI] [PubMed] [Google Scholar]
- Rosenblum (2014).Rosenblum WI. Why Alzheimer trials fail: removing soluble oligomeric beta amyloid is essential, inconsistent, and difficult. Neurobiology of Aging. 2014;35:969–974. doi: 10.1016/j.neurobiolaging.2013.10.085. [DOI] [PubMed] [Google Scholar]
- Schägger (2006).Schägger H. Tricine-SDS-PAGE. Nature Protocols. 2006;1:16–22. doi: 10.1038/nprot.2006.4. [DOI] [PubMed] [Google Scholar]
- Schneider et al. (2014).Schneider LS, Mangialasche F, Andreasen N, Feldman H, Giacobini E, Jones R, Mantua V, Mecocci P, Pani L, Winblad B, Kivipelto M. Clinical trials and late-stage drug development for Alzheimer’s disease: an appraisal from 1984 to 2014. Journal of Internal Medicine. 2014;275:251–283. doi: 10.1111/joim.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seneci (2015).Seneci P. Chemical modulators of protein misfolding and neurodegenerative disease. Amsterdam: Elsevier Academic Press; 2015. [Google Scholar]
- Studier (2005).Studier FW. Protein production by auto-induction in high density shaking cultures. Protein Expression and Purification. 2005;41:207–234. doi: 10.1016/j.pep.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Trivella et al. (2012).Trivella DBB, Dos Reis CV, Lima LMTR, Foguel D, Polikarpov I. Flavonoid interactions with human transthyretin: combined structural and thermodynamic analysis. Journal of Structural Biology. 2012;180:143–153. doi: 10.1016/j.jsb.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Ushikubo et al. (2012).Ushikubo H, Watanabe S, Tanimoto Y, Abe K, Hiza A, Ogawa T, Asakawa T, Kan T, Akaishi T. 3,3′,4′,5,5′-Pentahydroxyflavone is a potent inhibitor of amyloid β fibril formation. Neuroscience Letters. 2012;513:51–56. doi: 10.1016/j.neulet.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Vignaud et al. (2013).Vignaud H, Bobo C, Lascu I, Sörgjerd KM, Zako T, Maeda M, Salin B, Lecomte S, Cullin C. A structure-toxicity study of Aß42 reveals a new anti-parallel aggregation pathway. PLoS ONE. 2013;8:1–13. doi: 10.1371/journal.pone.0080262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh et al. (2009).Walsh DM, Thulin E, Minogue AM, Gustavsson N, Pang E, Teplow DB, Linse S. A facile method for expression and purification of the Alzheimer’s disease-associated amyloid β-peptide. FEBS Journal. 2009;276:1266–1281. doi: 10.1111/j.1742-4658.2008.06862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Dong & Sun (2012).Wang SH, Dong XY, Sun Y. Effect of (-)-epigallocatechin-3-gallate on human insulin fibrillation/aggregation kinetics. Biochemical Engineering Journal. 2012;63:38–49. doi: 10.1016/j.bej.2012.02.002. [DOI] [Google Scholar]
- Wobst et al. (2015).Wobst HJ, Sharma A, Diamond MI, Wanker EE, Bieschke J. The green tea polyphenol (−)-epigallocatechin gallate prevents the aggregation of tau protein into toxic oligomers at substoichiometric ratios. FEBS Letters. 2015;589:77–83. doi: 10.1016/j.febslet.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu et al. (1997).Zhu QY, Zhang A, Tsang D, Huang Y, Chen Z-Y. Stability of green tea catechins. Journal of Agricultural and Food Chemistry. 1997;45:4624–4628. doi: 10.1021/jf9706080. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Raw data
Raw data