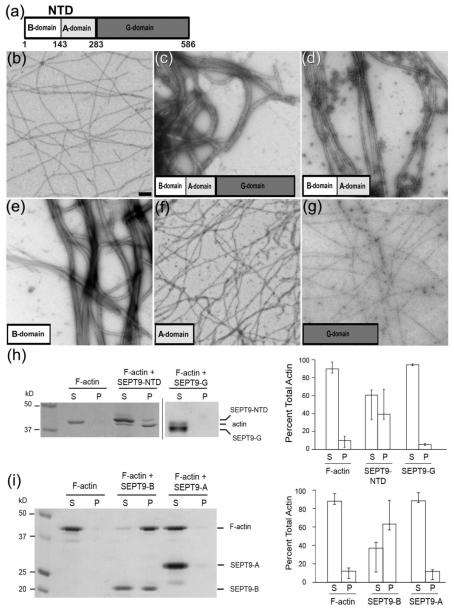
Figure 1.
SEPT9 bundles F-actin through the B-domain of its NTD. (a) - Schematic representation of SEPT9 domains. In addition to the G-domain found in all septin paralogs, SEPT9 has a unique N-terminal domain (NTD) consisting of a basic domain (B-domain) and an acidic domain (A-domain). (b) - Pure F-actin, scale bar: 1 nm. (c) - Full length SEPT9 efficiently bundles actin filaments within 2 minutes of incubation. (d) - NTD consisting of the B- and A-domains packs F-actin into tight bundles. (e) – The B-domain bundles F-actin into tight bundles morphologically similar to those shown in (d). (f) - The A-domain does not bind or bundle F-actin. (g) – The G-domain does not bundle F-actin. (h-i) – Low-speed sedimentation of F-actin in the presence of full length SEPT9 and its domains. Coomassie-stained gels with the corresponding bar graphs show that F-actin is recovered in the pellet in the presence of the NTD (h, SEPT9-NTD) and the B-domain (i, SEPT9-B). Error bars correspond to the maximum and minimum values from the independent experiments. In the presence of the G-domain (h, SEPT9-G) and the A-domain (i, SEPT9-A) most of the actin is recovered in the supernatant.