Fig. 1.
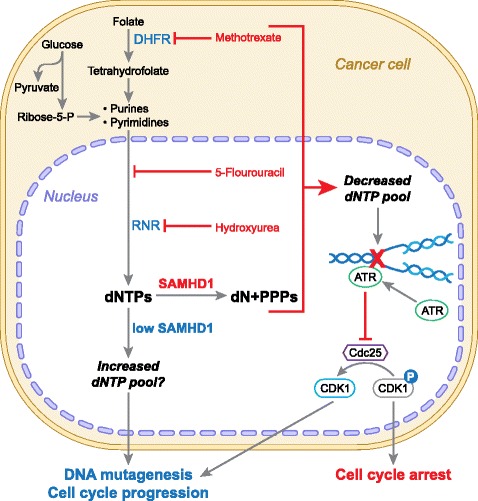
Dysregulation of dNTPs in cancer pathogenesis and its targeted therapy. Nucleotides are derived from multiple intracellular sources, including products of glycolysis, folate cycle, and scavenging of degraded components. The reduction of dihydrofolate to active tetrahydrofolate is inhibited by the chemotherapeutic methotrexate. Pyrimidine and purine bases are both reduced to deoxynucleosides (dN) by ribonucleotide reductase (RNR). This reaction is inhibited by the chemotherapeutic hydroxyurea. Other steps in this reaction are inhibited by numerous nucleoside analogs (“antimetabolite” compounds) including 5-fluorouracil. These drugs function by limiting the deoxynucleoside triphosphate (dNTP) pool available for DNA synthesis and triggering the S-phase checkpoint via the action of ATR and Chk1, resulting in cell cycle arrest by inhibiting the activation of cyclin dependent kinase 1 (CDK1). A potentially critical regulator of this pathway is SAMHD1, which hydrolyses dNTPs into products that are then recycled or degraded. By this action, SAMHD1 limits dNTP pool in G1 phase and prevents DNA replication. With loss of function or repression of SAMHD1 expression, the dNTP pool is not reduced which can result in DNA damage and inappropriate cell cycle progression. DHFR, dihydrofolate reductase; PPPs, triphosphate; ATR, ataxia-telangiectasia and Rad3-related protein; Cdc25, cell division cycle 25
