Abstract
In this article, we aimed to review the literature on the clinics and management of intraductal papillary mucinous neoplasm (IPMN). Intraductal papillary mucinous neoplasm of the pancreas is a mucin-producing cystic mass originating from the pancreatic ductal system. Approximately 25% of the pancreatic neoplasms resected surgically and 50% of pancreatic cysts detected incidentally are IPMNs. They can be benign or malignant in character, while malignant transformation of benign forms can be encountered. It is important to determine IPMNs in the early stages, implementation of appropriate treatment approaches, and follow-up to provide better prognosis. We reviewed the studies published in the English medical literature through PubMed and summarized the clinical features and current approaches to the treatment and follow-up of the IPMN. Due to the recent advances and widespread implementation of radiological imaging techniques, the incidental detection rate of IPMNs has increased significantly. The effective treatment of the disease is possible via the detailed diagnosis of the disease, determination of the prognostic factors, and a multidisciplinary approach. Recent literature also emphasized the molecular profile determination approaches for assessment of prognosis of patients with IPMN. Current knowledge on IPMN, a clinically important epidemiologic problem, shows that the treatment should be personalized considering the prognostic features and life expectancy of the patient.
Key words: Intraductal papillary mucinous neoplasm, Pancreas, Treatment options
Intraductal papillary mucinous neoplasm (IPMN) of the pancreas is a mucin-producing cystic mass originating from the pancreatic ductal system.1,2 It was first defined by Ohashi et al3 in 1982 following the detection of four patients with puffiness in the Vater ampulla, dilated pancreatic ducts, and mucin secretion. In the following years, lesions with similar characteristics were reported under different names. Finally, the World Health Organization (WHO) classified the mucin-producing cystic masses of the pancreas into 2 groups: mucinous cystic neoplasm and IPMN.4,5 Recent advances and widespread implementation of radiological imaging techniques, as well as the identification and classification of the disease, has led to an increased detection rate and incidence of IPMNs. They can be benign or malignant in character, while malignant transformation of benign forms can be encountered. In general, compared with sporadic pancreatic adenocarcinomas, invasive IPMNs have a better prognosis. The overall survival 5 years after resection is reported to be 22% for invasive IPMNs and 11% for sporadic pancreatic adenocarcinomas.6,7 It is important to determine IPMNs, which are clinically important epidemiologic problems, in the early stages, for implementation of appropriate treatment approaches, and follow-up to provide better prognosis.
Epidemiology
It has been reported that the prevalence of the cystic pancreatic lesions, including IPMNs, were 13 to 20% among asymptomatic individuals. Approximately 25% of the surgically resected pancreatic neoplasms and 50% of the incidentally detected pancreatic cysts are IPMNs.8,9 The estimated IPMN incidence (2.04/100,000/y) being higher than that of the pancreatic cancer incidence (0.8/100,000/y) shows that IPMN is clinically epidemiologically important.1,10
Etiopathogenesis and Classification
According to WHO, IPMNs are generally defined histologically as mucin-producing, long, columnar epithelial cell lesions that cover the dilated pancreatic ducts with a papillary structure.5 They are differentiated from the mucinous cystic neoplasms by the lack of ovarian stromas and a direct exposure of the pancreatic ductal system.
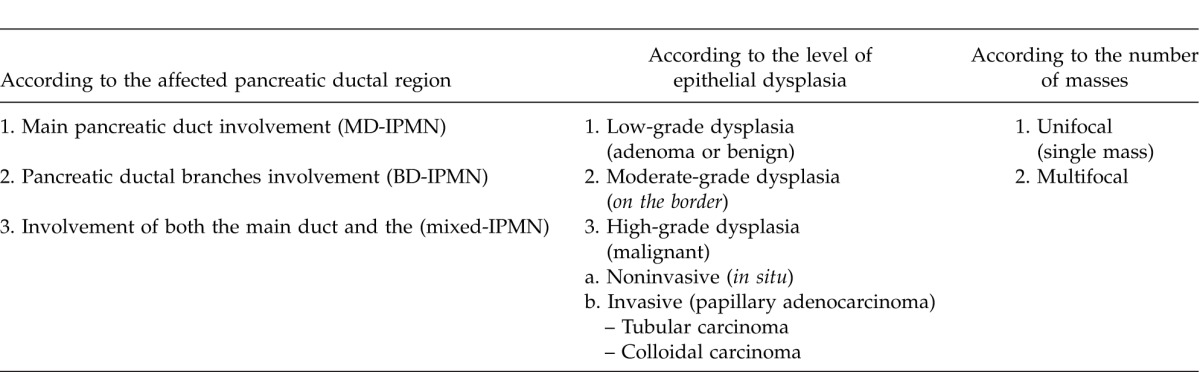
Intraductal papillary mucinous neoplasms are divided into three groups according to the pancreatic ductal sites they affect; IPMNs originated from the main pancreatic duct are called as main duct IPMN (MD-IPMN), whereas the ones originated from the ductal branches are called as branch duct IPMN (BD-IPMN); lastly, the others originating from both of them are called mixed-IPMN1,2 (Fig. 1). The most frequent type is the mixed-IPMN, whereas the least frequent one is the MD-IPMN type.11
Fig. 1.
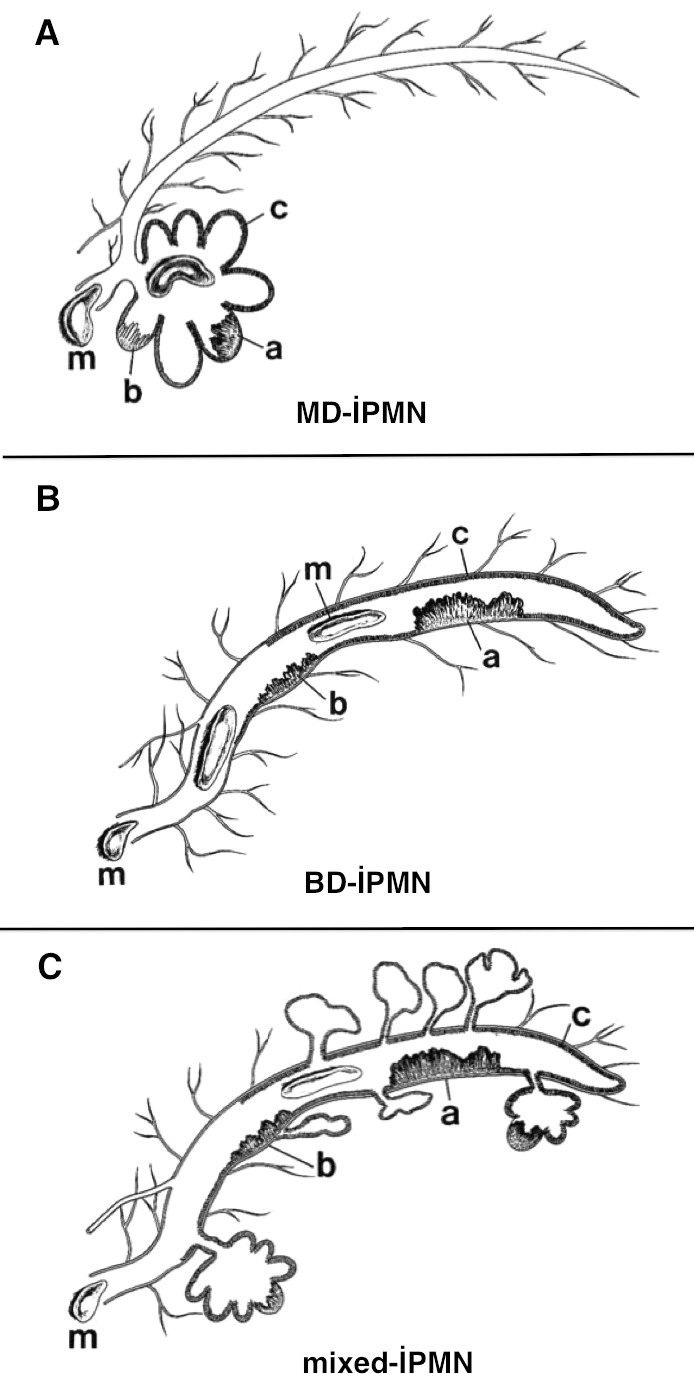
The schematic presentation of three IPMN types according to the affected pancreatic ductal region. (A) MD-IPMN. (B) BD-IPMN. (C) Mixed-IPMN. Main duct IPMN originates from the main pancreatic duct; BD-IPMN originates from the pancreatic ductal branches; and mixed-IPMN originates from both the main pancreatic duct and the ductal branches. Mucin formation is visualized within the duct. Examples of lesions according to the dysplasia types are shown in the figures as well. (a) Adenocarcinoma. (b) Adenoma. (c) Hyperplasia. (m) Mucin.
From the article of Lim et al. (2001), upon written permission of the author.
Pathologically, IPMNs may show different degrees of dysplasia that may extend to invasive carcinoma. According to the epithelial dysplasia grades, IPMNs may be classified as low-grade dysplasia (adenoma or benign), moderate grade dysplasia (border), and high-grade dysplasia (malignant). Malignant IPMNs may either be noninvasive (in situ) or invasive (papillary adenocarcinoma). Recently, invasive IPMNs are assessed in 2 sub-types as tubular carcinomas and colloidal carcinomas.
Other than their locations and pathological characteristics, IPMNs are divided into 2 groups: unifocal and multifocal. The intraductal papillary mucinous neoplasm may be a single cystic mass; however, approximately 14 to 40% of them are multifocal. Intraductal papillary mucinous neoplasms usually show multifocal characteristics in the elderly (≥68 years of age).1,2,11,12,13
Lately, the minimal involvement of main pancreatic duct is proposed to be categorized differently than extensive involvement of main pancreatic duct,14 but it has not gained common acceptance yet.
The classification of the IPMNs according to the most frequent classification methods are shown in Table 1.
Table 1.
The classification of IPMNs according to the most frequent classification methods
Clinical Appearance
Most of the patients with IPMN are asymptomatic for at least 1 year before diagnosis.15,16 When the disease becomes symptomatic, the most frequent clinical findings are stomachache, weight loss, steatorrhea, diarrhea, diabetes, pancreatitis, and jaundice.2,17 Physical examination may reveal a palpable hard mass in the abdomen.
Diagnosis
The diagnosis of IPMN is difficult since there is no golden standard method and the amount of the cystic fluid to be analyzed is limited. The diagnosis and grading should be implemented with different imaging techniques, plus the analysis of the cystic fluid and cytology.
Endoscopy
In patients with symptoms of obstructive jaundice, endoscopic retrograde cholangiopancreatography (ERCP) is used in the early diagnosis.18 The pathognomonic finding, observed in 55% of the patients with IPMN, is an image of mucin secretion from the swollen papilla. Magnetic resonance imaging (MRI) cholangiopancreatography is a good alternative to ERCP with its noninvasive and repeatability features. Both methods were shown to be comparable and show similar diagnostic accuracy rates in IPMN.
Endoscopic ultrasonography (USG), on the other hand, is a preferred method in the diagnosis and classification of IPMN since it enables fine needle aspiration for cytological examination. It may provide detailed information on the tumor and its surroundings. Since the endoscopic USG has a lower rate of complication than ERCP and provides the opportunity for cytological examination, molecular analysis, analysis of the tumor markers and enzyme levels from the pancreatic fluid obtained by aspiration, it is the most commonly preferred endoscopic method in the diagnosis of IPMNs.
Radiology
Abdominal computed tomography (CT) is the widest used modality in the diagnosis of IPMN. The computed tomography scan reveals the cystic, septal, multifocal lesions of the pancreas that may include a solid component as well. The pancreatic duct may not necessarily be visualized.17 However, a pancreatic duct dilatation due to high amounts of mucin production may be observed radiologically.19 When parenchymal atrophy accompanies this image, it indicates a ductal obstruction and subsequent organ failure and dysfunction. Two-dimensional inclined CT images obtained from the main pancreatic duct may demonstrate the potential communication pathways between the main duct and its branches.20 The presence of hepatic metastasis in the CT is in favor of malignant transformation. Care should be taken to get arterial and venous phase images with thin slices (2–3 mm) from the whole pancreas for the CT evaluation of pancreatic masses.
Multislice CT applications and 2-dimensioned coronal, sagittal and 3-dimensioned vascular reconstructions may help to diagnose the localization of the lesion with vascular involvement and malignancy indicators with higher accuracy.19
Positron emission tomography (PET)-CT is known to have benefits in the diagnosis, grading, and treatment of pancreatic adenocarcinomas.21 However, its role in the diagnosis of the malignancies in cystic pancreatic lesions is controversial, and more studies are needed to reveal the subject.22
Tumor markers
It has been demonstrated that the increased carcinoembryonic antigen (CEA) level (>192 ng/mL) within the cystic fluid is an indicator of mucinous neoplasm. Although it has been accepted that high levels of CA 19-9 in the serum (>35 U/mL) may be an indicator of pancreatic biliary malignancy, it has been observed that its levels within the cystic fluid has no effect on the discrimination of mucinous and nonmucinous tumor types. The amylase level in the cystic fluid on the other hand, may have benefits in the discrimination of the pseudocysts, and mucinous or serosal cystic neoplasms: a slight increase in the amylase level substantially eliminates the presence of a pseudocyst. In extremely high levels, it should be evaluated in conjunction with other indicators for the diagnosis of IPMN.23
Prognosis
Recent findings demonstrate that progression from low-grade dysplasia toward high-grade dysplasia is present,24,25 and 30 to 50% of the patients diagnosed with IPMN had invasive carcinoma.26–28 However, the rate of this progression is not yet defined. In many cases, different grades of dysplasia—in other words, different malignant transformation levels—may be detected within the different sites of the same surgical specimen. Malignant transformation is usually accompanied by some genetic mutations.11,29,30 In a recent meta-analysis, hTERT expression was demonstrated to be strongly related to the malignant transformation of IPMN, and although frequently observed in malignant IPMNs, kRas and MUC5AC expressions were demonstrated not to be strongly related to the transformation.31
The risk of malignancy is higher in patients with MD-IPMN or mixed-IPMN than the BD-IPMN type (70 versus 25%, respectively).1,2,32 Certain radiological features of the tumor are valuable for the determination of the malignancy risk. The presence of mural nodules, cystic wall thickness, growth of the surrounding lymph nodes, and cholestasis are indicators of malignant transformation of the lesion.33,34 But rarely concomitant adenocarcinoma may be seen with branch duct intraductal papillary mucinous neoplasms.35
The 5-year postsurgical survival rate is reported to be 77 to 100% for benign/preinvasive IPMN and 22 to 65% for malignant/invasive IPMN.24,36–40 In patients who underwent a pancreatectomy due to IPMN, the 5-year-survival rate was 89 to 95% for benign IPMNs, whereas it was 63 to 65% for malignant IPMNs.41–43 Colloidal carcinomas among the invasive IPMNs have higher 5-year-survival rates than tubular carcinomas (57–83% and 24–55%, respectively).44,45
Postsurgical prognosis is claimed to be related to some pathological indicators. These include tumor size, lymph node involvement, tumor grade, percentage of the invasive junction, and vascular and perineural involvement.46–51
It is reported that multifocal IPMNs are more frequently located in the caudal part of the pancreas with higher serum tumor marker (CA 19-9/CEA) levels, and higher risk of malignancy.52 The risk of malignant transformation is reported to be 58.8% for multifocal IPMN and 49.2% for unilocal IPMN.
Treatment Options
The risk of malignancy is higher in symptomatic patients than in asymptomatic patients, and in MD-IPMN or mixed-IPMN than in BD-IPMN type.1,2 Therefore, in patients with symptomatic IPMNs with MD-IPMN or mixed-IPMN, the tumor should primarily be surgically removed when possible.
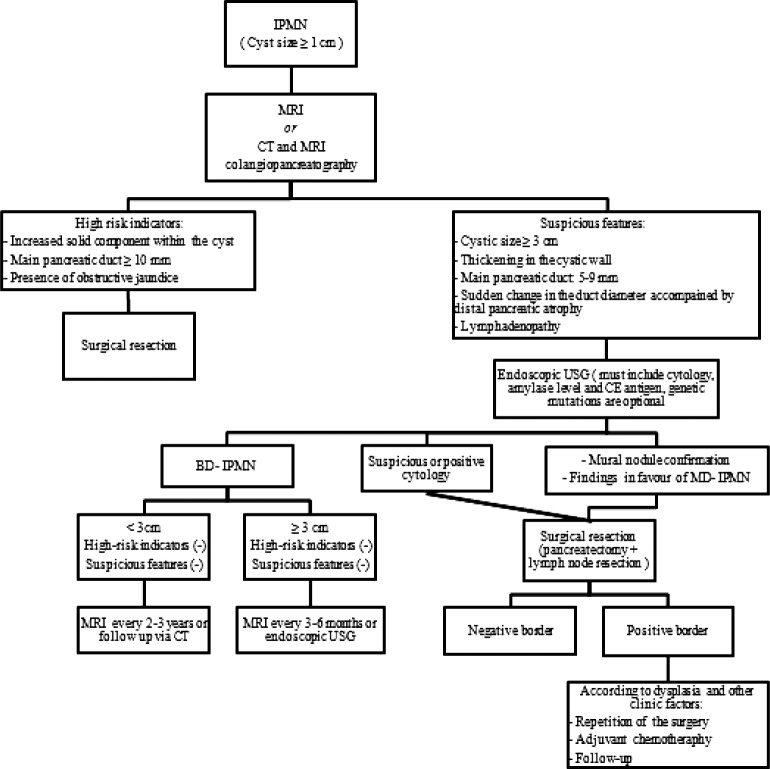
According to the guidelines in the International Consensus on the Diagnosis and Treatment of IPMN, which was first established by Tanaka et al34 in 2006 and updated in 2012, the approach to patients with IPMN is schematized in Fig. 2. These guidelines recommend the usage of MRI or CT, and MRI cholangiopancreatography in patients with cysts larger than or equal to 1 cm in order to determine the risk ratios of these cysts. According to the results of these examinations, patients carrying the indicators of a high risk as solid component, main pancreatic duct width larger than 10 mm, or the presence of obstructive jaundice should be taken to surgical resection. For the cysts with suspicious characteristics like: cystic size bigger than 3 cm, thickening in the cystic wall, presence of a mural nodule, main pancreatic duct width of 5 to 9 mm, sudden change in the diameter of the main duct accompanied by distal pancreatic atrophy and lymphadenopathy, endoscopic USG should be performed including cystic fluid aspiration and analysis. Patients with cyst sizes larger than 3 cm, those who are confirmed to have mural nodules by endoscopic USG, those who have suspicious or positive cytology, and those with findings in favor of MD-IPMN should undergo surgical resection as well. The surgical resection should include pancreatectomy and lymph node resections.
Fig. 2.
The schematized presentation of approach to patients with IPMN, according to the guidelines of the 2012 International Consensus on the Diagnosis and Treatment of IPMN.
Patients who are found to have BD-IPMN radiologically should be monitored every 3 to 6 months when the cyst is larger than 3 cm, and once every 2 to 3 years when the cyst is smaller than this size.1 Asymptomatic and multifocal BD-IPMNs may be monitored at intervals determined according to the size and morphology of the cyst.53 However, it should be kept in mind that these patients carry the risk of malignant transformation during the clinical follow-up period; thus, a lifelong clinical follow-up should be planned, and a surgical intervention should certainly be performed in the presence of any suspicion. In a retrospective study that evaluated BD-IPMN patients who underwent pancreatectomy, no difference was recorded for patients with solitary and multifocal IPMNs regarding pathological malignancy rates and they recommended surgical intervention for only the patients with malignant risk factors among those carrying the multifocal BD-IPMN type, and a close monitoring for the remaining.54
Surgical border positivity was shown to be related to recurrence in all patients with IPMN, whereas lymph node metastasis was shown to be related to invasive IPMN.55 Therefore, obtaining a negative border is important for an effective surgical resection. In pancreatectomy cases that are performed for IPMNs, the definition of positive resection margin is controversial and this leads to heterogenous results among studies.
In some studies, normal epithelium or mucinous hyperplasia without dysplasia in the main duct are indications of negative surgical margins; adenoma, borderline neoplasm, or carcinoma are indications of positive surgical margins56; while in others, the classification which consist of negative resection margin (with normal columnar epithelium or deluded), mucinous hyperplasia (pancreatic intraepithelial neoplasia Pan-IN 1A or Pan 1B), and positive resection margin (dysplasia Pan-IN 2 or carcinoma Pan-IN 3) is used.57,58
When the degree of dysplasia is considered; some studies report negative surgical margin if normal epithelium or IPMN adenoma is present, and positive if moderate or severe dysplasia is present (borderline IPMN or carcinoma in situ IPMN).59 Otherwise, if at least IPMN adenoma on the main duct or at least borderline IPMN on branch ducts is present, it can be defined as “significant” and requires additional resection.60
Narrow band imaging and intraoperative pancreatoscopy is recommended in particular cases.61 However, further studies are required on the subject.
There is limited information on the course and postoperative treatment approaches to the surgically resected IPMN. There are studies demonstrating the development of recurrent lesions in the remaining pancreas within 3 to 6 years following the operations of patients for noninvasive IPMN.62,63 Caponi et al64 have demonstrated that lymph node negative patients with invasive IPMN, including well to moderately differentiated tumor cells, had a longer postoperative general survival rate. It was also demonstrated in the study that adjuvant chemotherapy including gemcitabine was effective in extending the survival periods. Similarly, adjuvant 5-fluorouracil with radiotherapy was shown to increase the survival rate when compared with surgery alone.65 However, there are also studies suggesting there is no advantage to adjuvant chemotherapy in terms of survival.48 This difference may be due to the differences in the prognostic factors between the series. It seems that in invasive IPMN patients with good prognosis according to the tumor grade and lymph node involvement, postoperative adjuvant therapy provides benefits.
Since data concerning the malignant progression pattern of the disease are limited, the aggressive and preventive approaches in a treatment should be equilibrated and a multidisciplinary and patient-specific treatment approach should be implemented with the assessment of all prognostic factors.23 It is demonstrated that the comorbidity and the age of the patient at the time of diagnosis with IPMN are the factors that affect mortality, independent from the pancreas cancer.66 Therefore, close follow-up may be considered for elderly patients with comorbidity instead of surgery. In the series of Baiocchi et al,67 including 4943 patients, 61.1% of the patients met the surgical resection criteria; however, it was performed in only 17.9%. The remaining 67.2% were followed-up. Despite this preventive approach, malignancy was observed in only 2.4% of the patients during a median duration of 39 months.
Conclusion
Due to the recent advances and widespread implementation of radiological imaging techniques, the incidental detection rate of IPMNs has increased significantly. Approximately 25% of the pancreatic neoplasms resected surgically and 50% of pancreatic cysts detected incidentally are IPMNs. They can be benign or malignant in character, while malignant transformation of benign forms can be encountered. In general, compared with malignant pancreatic ductal adenocarcinoma, IPMNs have better prognosis. The effective treatment of the disease is possible via the detailed diagnosis of the disease, determination of the prognostic factors and a multidisciplinary approach. The treatment should be personalized considering the prognostic features and life expectancy of the patient. A more appropriate treatment will be possible soon in the future by the molecular profile determination approaches emphasized recently.
Acknowledgments
We thank Dr. Jae Hoon Lim for permitting us to use the illustrations in Fig. 1 in this review.
References
- 1.Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12(3):183–197. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Crippa S, Fernández-Del Castillo C, Salvia R, Finkelstein D, Bassi C, Domínguez I, et al. Mucin-producing neoplasms of the pancreas: an analysis of distinguishing clinical and epidemiologic characteristics. Clin Gastroenterol Hepatol. 2010;8(2):213–219. doi: 10.1016/j.cgh.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohashi K, Murakami Y, Takekoshi T. Four cases of mucin producing cancer of the pancreas on specific findings of papilla of vater. Prog Diag Endosc. 1982;20:348–51. [Google Scholar]
- 4.Zamboni G, Kloppel G, Hruban RH, Longnecker DS, Adler G. Mucinous cystic neoplasms of the pancreas. In: SR Hamilton, Aaltonen LA., editors. World Health Organization Classification of Tumors. Lyon, France: IARC Press; 2000. pp. 234–236. In. eds. [Google Scholar]
- 5.Longnecker DS, Adler G, Hruban RH. Intraductal papillarymucinous neoplasms of the pancreas. In: SR Hamilton, Aaltonen LA., editors. World Health Organization Classification of Tumors. Lyon, France: IARC Press; 2000. pp. 237–241. In. eds. [Google Scholar]
- 6.Wasif N, Bentrem DJ, Farrell JJ, Ko CY, Hines OJ, Reber HA. Invasive intraductal papillary mucinous neoplasm versus sporadic pancreatic adenocarcinoma: a stage-matched comparison of outcomes. Cancer. 2010;116(14):3369–3377. doi: 10.1002/cncr.25070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waters JA, Schnelldorfer T, Aguilar-Saavedra JR, Chen JH, Yiannoutsos CT, Lillemoe KD. Survival after resection for invasive intraductal papillary mucinous neoplasm and for pancreatic adenocarcinoma: a multi-institutional comparison according to American Joint Committee on cancer stage. J Am Coll Surg 201; 213(2):275–283. doi: 10.1016/j.jamcollsurg.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Andrejevic-Blant S, Kosmahl M, Sipos B, Klöppel G. Pancreatic intraductal papillary-mucinous neoplasms: a new and evolving entity. Virchows Arch. 2007;451(5):863–869. doi: 10.1007/s00428-007-0512-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kosmahl M, Pauser U, Peters K, Sipos B, Lüttges J, Kremer B. Cystic neoplasms of the pancreas and tumor-like lesions with cystic features: a review of 418 cases and a classification proposal. Virchows Arch. 2004;445(2):168–178. doi: 10.1007/s00428-004-1043-z. [DOI] [PubMed] [Google Scholar]
- 10.Lee KS, Sekhar A, Rofsky NM, Pedrosa I. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol. 2010;105(9):2079–2084. doi: 10.1038/ajg.2010.122. [DOI] [PubMed] [Google Scholar]
- 11.Fritz S, Fernandez-del Castillo C, Mino-Kenudson M, Crippa S, Deshpande V, Lauwers GY, et al. Global genomic analysis of intraductal papillary mucinous neoplasms of the pancreas reveals significant molecular differences compared to ductal adenocarcinoma. Ann Surg. 2009;249(3):440–447. doi: 10.1097/SLA.0b013e31819a6e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthaei H, Norris AL, Tsiatis AC, Olino K, Hong SM, dal Molin M, et al. Clinicopathological characteristics and molecular analyses of multifocal intraductal papillary mucinous neoplasms of the pancreas. Ann Surg. 2012;255(2):326–233. doi: 10.1097/SLA.0b013e3182378a18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pelaez-Luna M, Chari ST, Smyrk TC, Takahashi N, Clain JE, Levy MJ. Do consensus indications for resection in branch duct intraductal papillary mucinous neoplasm predict malignancy? A study of 147 patients. Am J Gastroenterol. 2007;102(8):1759–1764. doi: 10.1111/j.1572-0241.2007.01224.x. [DOI] [PubMed] [Google Scholar]
- 14.Sahora K, Fermandez-del Castillo C, Dong F, Marchegiani G, Thayer SP, Ferrone CR, et al. Not all mixed-type intraductal papillary mucinous neoplasms behave like main-duct lesions: Implications of minimal involvement of the main pancreatic duct. Surgery. 2014;156(3):611–621. doi: 10.1016/j.surg.2014.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khalid A, McGrath KM, Zahid M, Wilson M, Brody D, Swalsky P. The role of pancreatic cyst fluid molecular analysis in predicting cyst pathology. Clin Gastroenterol Hepatol. 2005;3(10):967–973. doi: 10.1016/s1542-3565(05)00409-x. [DOI] [PubMed] [Google Scholar]
- 16.Taouli B, Vilgrain V, O'Toole D, Vullierme MP, Terris B, Menu Y. Intraductal papillary mucinous tumors of the pancreas: features with multimodality imaging. J Comput Assist Tomogr. 2002;26(2):223–231. doi: 10.1097/00004728-200203000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Crippa S, Partelli S, Tamburrino D, Falconi M. The natural history of a branch-duct intraductal papillary mucinous neoplasm of the pancreas. Surgery. 2014;155(3):578–579. doi: 10.1016/j.surg.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Lim JH, Lee G, Oh YL. Radiologic spectrum of intraductal papillary mucinous tumor of the pancreas. Radiographics. 2001;21(2):323–337. doi: 10.1148/radiographics.21.2.g01mr01323. [DOI] [PubMed] [Google Scholar]
- 19.Kawamoto S, Horton KM, Lawler LP, Hruban RH, Fishman EK. Intraductal papillary mucinous neoplasm of the pancreas: can benign lesions be differentiated from malignant lesions with multidetector CT? Radiographics. 2005;25(6):1451–1468. doi: 10.1148/rg.256055036. [DOI] [PubMed] [Google Scholar]
- 20.Fukukura Y, Fujiyoshi F, Sasaki M, Inoue H, Yonezawa S, Nakajo M. Intraductal papillary mucinous tumors of the pancreas: thin-section helical CT findings. AJR Am JS Roentgenol. 2000;174(2):441–447. doi: 10.2214/ajr.174.2.1740441. [DOI] [PubMed] [Google Scholar]
- 21.Heinrich S, Goerres GW, Schäfer M, Sagmeister M, Bauerfeind P, Pestalozzi BC. Positron emission tomography/computed tomography influences on the management of resectable pancreatic cancer and its cost-effectiveness. Ann Surg. 2005;242(2):235–243. doi: 10.1097/01.sla.0000172095.97787.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sperti C, Bissoli S, Pasquali C, Frison L, Liessi G, Chierichetti F. 18-fluorodeoxyglucose positron emission tomography enhances computed tomography diagnosis of malignant intraductal papillary mucinous neoplasms of the pancreas. Ann Surg. 2007;246(6):932–937. doi: 10.1097/SLA.0b013e31815c2a29. [DOI] [PubMed] [Google Scholar]
- 23.Nair RM, Barthel JS, Centeno BA, Choi J, Klapman JB, Malafa MP. Interdisciplinary management of an intraductal papillary mucinous neoplasm of the pancreas. Cancer Control. 2008;15(4):322–333. doi: 10.1177/107327480801500407. [DOI] [PubMed] [Google Scholar]
- 24.Salvia R, Fernández-del Castillo C, Bassi C, Thayer SP, Falconi M, Mantovani W, et al. Main duct intraductal papillary-mucinous neoplasms of the pancreas: clinical predictors of malignancy and long term survival following resection. Ann Surg. 2004;239(5):678–685. doi: 10.1097/01.sla.0000124386.54496.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sohn TA, Yeo CJ, Cameron JL, Hruban RH, Fukushima N, Campbell KA. Intraductal papillary-mucinous neoplasms of the pancreas: an update experience. Ann Surg. 2004;239(6):788–797. doi: 10.1097/01.sla.0000128306.90650.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimura W, Makuuchi M, Kuroda A. Characteristics and treatment of mucin-producing tumor of the pancreas. Hepatogastroenterology. 1998;45(24):2001–2008. [PubMed] [Google Scholar]
- 27.Yamao K, Ohashi K, Nakamura T, Suzuki T, Shimizu Y, Nakamura Y. The prognosis of intraductal papillary-mucinous tumors of the pancreas. Hepatogastroenterology. 2000;47(34):1129–1134. [PubMed] [Google Scholar]
- 28.Basturk O, Coban I, Adsay NV. Pancreatic cysts: pathologic classification, differential diagnosis, and clinical implications. Arch Pathol Lab Med. 2009;133(3):423–438. doi: 10.5858/133.3.423. [DOI] [PubMed] [Google Scholar]
- 29.Hruban RH, Adsay NV. Molecular classification of neoplasms of the pancreas. Hum Pathol. 2009;40(5):612–623. doi: 10.1016/j.humpath.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Z'graggen K, Rivera JA, Compton CC, Pins M, Werner J, Fernández-del Castillo C, et al. Prevalence of activating K-ras mutations in the evolutionary stages of neoplasia in intraductal papillary mucinous tumors of the pancreas. Ann Surg. 1997;226(4):491–498. doi: 10.1097/00000658-199710000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nissim S, Idos GE, Wu B. Genetic markers of malignant transformation in intraductal papillary mucinous neoplasm of the pancreas: a meta-analysis. Pancreas. 2012;41(8):1195–1205. doi: 10.1097/MPA.0b013e3182580fb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D'Souza MA, Isaksson B, Löhr M, Enochsson L, Swahn F, Lundell L. The clinicopathological spectrum and management of intraductal papillary mucinous neoplasm of the bile duct (IPMN-B) Scand J Gastroenterol. 2013;48(4):473–479. doi: 10.3109/00365521.2012.722672. [DOI] [PubMed] [Google Scholar]
- 33.Sahani DV, Kadavigere R, Blake M, Fernández-Del Castillo C, Lauwers GY, Hahn PF. Intraductal papillary mucinous neoplasm of pancreas: multi-detector row CT with 2D curved reformations—correlation with MRCP. Radiology. 2006;238(2):560–569. doi: 10.1148/radiol.2382041463. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka M, Chari S, Adsay V, Fernández-del Castillo C, Falconi M, Shimizu M, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6(1–2):17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 35.Law JK, Wolfgang CL, Weiss MJ, Lennon AM. Concomitant pancreatic adenocarcinoma in a patient with branch-duct intraductal papillary mucinous neoplasm. World J Gastroenterol. 2014;20(27):9200–9204. doi: 10.3748/wjg.v20.i27.9200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez JR, Salvia R, Crippa S, Warshaw AL, Bassi C, Falconi M. Branch-duct intraductal papillary mucinous neoplasms: observations in 145 patients who underwent resection. Gastroenterology. 2007;133(1):72–79. doi: 10.1053/j.gastro.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Partelli S, Fernández-Del Castillo C, Bassi C, Mantovani W, Thayer SP, Crippa S, et al. Invasive intra-ductal papillary mucinous carcinomas of the pancreas: predictors of survival and the role of lymph node ratio. Ann Surg. 2010;251(3):477–482. doi: 10.1097/SLA.0b013e3181cf9155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schnelldorfer T, Sarr MG, Nagorney DM, Zhang L, Smyrk TC, Qin R. Experience with 208 resections for intraductal papillary mucinous neoplasm of the pancreas. Arch Surg. 2008;143(7):639–646. doi: 10.1001/archsurg.143.7.639. [DOI] [PubMed] [Google Scholar]
- 39.Nagai K, Doi R, Kida A, Kami K, Kawaguchi Y, Ito T. Intraductal papillary mucinous neoplasms of the pancreas: clinicopathologic characteristics and long-term follow-up after resection. World J Surg. 2008;32(2):271–278. doi: 10.1007/s00268-007-9281-2. [DOI] [PubMed] [Google Scholar]
- 40.Niedergethmann M, Grützmann R, Hildenbrand R, Dittert D, Aramin N, Franz M. Outcome of invasive and noninvasive intraductal papillary-mucinous neoplasms of the pancreas (IPMN): a 10-year experience. World J Surg. 2008;32(10):2253–2260. doi: 10.1007/s00268-008-9692-8. [DOI] [PubMed] [Google Scholar]
- 41.Jang JY, Kim SW, Ahn YJ, Yoon YS, Choi MG, Lee KU. Multicenter analysis of clinicopathologic features of intraductal papillary mucinous tumor of the pancreas: is it possible to predict the malignancy before surgery? Ann Surg Oncol. 2005;12(2):124–132. doi: 10.1245/ASO.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 42.Murakami Y, Uemura K, Hayashidani Y, Sudo T, Sueda T. Predictive factors of malignant or invasive intraductal papillary-mucinous neoplasms of the pancreas. J Gastrointest Surg. 2007;11(3):338–344. doi: 10.1007/s11605-006-0069-8. [DOI] [PubMed] [Google Scholar]
- 43.Shin SH, Han DJ, Park KT, Kim YH, Park JB, Kim SC. Validating a simple scoring system to predict malignancy and invasiveness of intraductal papillary mucinous neoplasms of the pancreas. World J Surg. 2010;34(4):776–783. doi: 10.1007/s00268-010-0416-5. [DOI] [PubMed] [Google Scholar]
- 44.Adsay NV, Pierson C, Sarkar F, Abrams J, Weaver D, Conlon KC. Colloid (mucinous noncystic) carcinoma of the pancreas. Am J Surg Pathol. 2001;25(1):26–42. doi: 10.1097/00000478-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 45.Sadakari Y, Ohuchida K, Nakata K, Ohtsuka T, Aishima S, Takahata S. Invasive carcinoma derived from the nonintestinal type intraductal papillary mucinous neoplasm of the pancreas has a poorer prognosis than that derived from the intestinal type. Surgery. 2010;147(6):812–817. doi: 10.1016/j.surg.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 46.D'Angelica M, Brennan MF, Suriawinata AA, Klimstra D, Conlon KC. Intraductal papillary mucinous neoplasms of the pancreas: an analysis of clinicopathologic features and outcome. Ann Surg. 2004;239(3):400–408. doi: 10.1097/01.sla.0000114132.47816.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Conlon KC. Intraductal papillary mucinous tumors of the pancreas. J Clin Oncol. 2005;23(20):4518–4523. doi: 10.1200/JCO.2005.22.517. [DOI] [PubMed] [Google Scholar]
- 48.Turrini O, Waters JA, Schnelldorfer T, Lillemoe KD, Yiannoutsos CT, Farnell MB. Invasive intraductal papillary mucinous neoplasm: predictors of survival and role of adjuvant therapy. HPB (Oxford) 2010;12(7):447–455. doi: 10.1111/j.1477-2574.2010.00196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilentz RE, Hruban RH. Pathology of cancer of the pancreas. Surg Oncol Clin N Am. 1998;7(1):43–65. [PubMed] [Google Scholar]
- 50.Jang JY, Hwang DW, Kim MA, Kang MJ, Lim CS, Lee SE. Analysis of prognostic factors and a proposed new classification for invasive papillary mucinous neoplasms. Ann Surg Oncol. 2011;18(3):644–650. doi: 10.1245/s10434-010-1331-6. [DOI] [PubMed] [Google Scholar]
- 51.Jang JW, Kim MH, Jeong SU, Kim J, Park do H, Lee SS, et al. Clinical characteristics of intraductal papillary mucinous neoplasm manifesting as acute pancreatitis or acute recurrent pancreatitis. J Gastroenterol Hepatol. 2013;28(4):731–738. doi: 10.1111/jgh.12121. [DOI] [PubMed] [Google Scholar]
- 52.Fritz S, Schirren M, Klauss M, Bergmann F, Hackert T, Hartwig W. J. Clinicopathologic characteristics of patients with resected multifocal intraductal papillary mucinous neoplasm of the pancreas. Surgery. 2012;152(3 suppl 1):S74–S80. doi: 10.1016/j.surg.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 53.Tanno S, Nakano Y, Nishikawa T, Nakamura K, Sasajima J, Minoguchi M. Natural history of branch duct intraductal papillary-mucinous neoplasms of the pancreas without mural nodules: long-term follow-up results. Gut. 2008;57(3):339–343. doi: 10.1136/gut.2007.129684. [DOI] [PubMed] [Google Scholar]
- 54.Mori Y, Ohtsuka T, Kono H, Ideno N, Aso T, Nagayoshi Y. Management strategy for multifocal branch duct intraductal papillary mucinous neoplasms of the pancreas. Pancreas. 2012;41(7):1008–1012. doi: 10.1097/MPA.0b013e31824b22c6. [DOI] [PubMed] [Google Scholar]
- 55.Leng KM, Wang ZD, Zhao JB, Cui YF, Zhong XY. Impact of pancreatic margin status and lymph node metastases on recurrence after resection for invasive and noninvasive intraductal papillary mucinous neoplasms of the pancreas: a meta-analysis. Dig Surg. 2012;29(3):213–225. doi: 10.1159/000339334. [DOI] [PubMed] [Google Scholar]
- 56.Crippa S, Partelli S, Falconi M. Extent of surgical resections for intraductal papillary mucinous neoplasms. World J Gastrointest Surg. 2010;2(10):347–351. doi: 10.4240/wjgs.v2.i10.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chari ST, Yadav D, Smyrk TC, DiMagno EP, Miller LJ. Study of recurrence after surgical resection of intraductal papillary mucinous neoplasm of the pancreas. Gastroenterology. 2002;123(5):1500–1507. doi: 10.1053/gast.2002.36552. [DOI] [PubMed] [Google Scholar]
- 58.Hruban RH, Adsay NV, Albores-Saavedra J, Compton C, Garret ES. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol. 2001;25(5):579–586. doi: 10.1097/00000478-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 59.Eguchi H, Ishikawa O, Ohigashi H, Sasaki Y, Yamada T, Nakaizumi A. Role of intraoperative cytology combined with histology in detecting continuous and skip type intraductal cancer existence for intraductal papillary mucinous carcinoma of the pancreas. Cancer. 2006;107(11):2567–2575. doi: 10.1002/cncr.22301. [DOI] [PubMed] [Google Scholar]
- 60.Couelard A, Sauvanet S, Kianmanesh R, Hammel P, Coinot N, Levy O. Frozen sectioning of the pancreatic cut surface during resection of intraductal papillary mucinous neoplasms of the pancreas is useful and reliable: a prospective evaluation. Ann Surg. 2005;242(6):774–780. doi: 10.1097/01.sla.0000188459.99624.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yelamali A, Mansard MJ, Dama R, Rebela P, Rao GV, Reddy DN. Intraoperative pancreatoscopy with narrow band imaging: a novel method for assessment of resection margins in case of intraductal papillary mucinous neoplasm. Surg Endosc. 2012;26(12):3682–3685. doi: 10.1007/s00464-012-2365-6. [DOI] [PubMed] [Google Scholar]
- 62.Nagai E, Ueki T, Chijiiwa K, Tanaka M, Tsuneyoshi M. Intraductal papillary mucinous neoplasms of the pancreas associated with so-called “mucinous ductal ectasia.” Histochemical and immunohistochemical analysis of 29 cases. Am J Surg Pathol. 1995;19(5):576–589. doi: 10.1097/00000478-199505000-00010. [DOI] [PubMed] [Google Scholar]
- 63.Azar C, Van de Stadt J, Rickaert F, Devière M, Baize M, Klöppel G. Intraductal papillary mucinous tumours of the pancreas. Clinical and therapeutic issues in 32 patients. Gut. 1996;39(3):457–464. doi: 10.1136/gut.39.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Caponi S, Vasile E, Funel N, De Lio N, Campani D, Ginocchi L. Adjuvant chemotherapy seems beneficial for invasive intraductal papillary mucinous neoplasms. Eur J Surg Oncol. 2013;39(4):396–403. doi: 10.1016/j.ejso.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 65.Swartz MJ, Hsu CC, Pawlik TM, Winter J, Hruban RH, Guler M. Adjuvant chemoradiotherapy after pancreatic resection for invasive carcinoma associated with intra-ductal papillary mucinous neoplasm of the pancreas. Int J Radiat Oncol Biol Phys. 2010;76(3):839–844. doi: 10.1016/j.ijrobp.2009.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kawakubo K, Tada M, Isayama H, Sasahira N, Nakai Y, Takahara N. Risk for mortality from causes other than pancreatic cancer in patients with intraductal papillary mucinous neoplasm of the pancreas. Pancreas. 2013;42(4):687–691. doi: 10.1097/MPA.0b013e318270ea97. [DOI] [PubMed] [Google Scholar]
- 67.Baiocchi GL, Portolani N, Grazioli L, Mazza G, Gheza F, Bartoli M. Management of pancreatic intraductal papillary mucinous neoplasm in an academic hospital (2005–2010): What follow-up for unoperated patients? Pancreas. 2013;42(4):696–700. doi: 10.1097/MPA.0b013e318270b98b. [DOI] [PubMed] [Google Scholar]