Fig 3. Purification of inclusion bodies by solubilization and refolding in alkaline-based buffer containing redox agents.
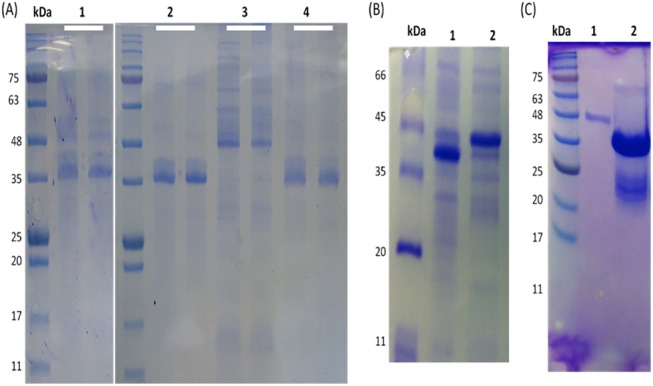
A) Semi-solubilization of inclusion bodies in dH2O (pH 12) after 10 min of incubation. L1, the isolated inclusion bodies; L2, purified protein (final product); L3, removing of host cell proteins; L4, remaining insoluble aggregates after final solubilization (each line duplicated). B) Refolding of soluble protein in buffer containing oxidized and reduced glutathione to reform the disulphide bridges of TACH. The refolded protein showed different levels under reducing and non-reducing conditions of the SDS-PAGE, indicating the formation of disulphide bridges. C) SDS-PAGE analysis of the fusion protein. L1, a single band at the expected size for the monomer. L2, double bands of the fusion protein. The upper faint band at the dimer size and the lower thick band at the monomer size (arrows).
