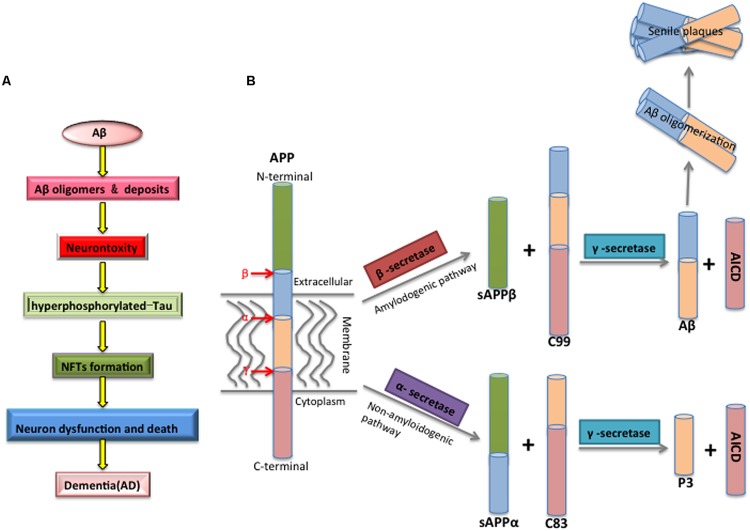
FIGURE 1.
(A) The mechanism of Aβ toxicity. Accumulating Aβ will initially results in Aβ oligomerization, gradually deposits as the forms of fibrils and senile plaques. Furthermore, Aβ aggregation alters the kinase/phosphatase activity that leads to the Tau protein hyperphosphorylated, which causes the formation of neurofibrillary tangles (NFTs), and eventual synaptic and neuronal dysfunction and AD. (B) The proteolytic processing of the amyloid precursor protein (APP) and Aβ biogenesis. APP is a transmembrane glycoprotein with a large luminal domain and a short cytoplasmic domain, and it is processed through amyloidogenic or non-amyloidogenic pathway. The amyloidogenic pathway is the process of Aβ biogenesis: APP is firstly cleaved by β-secretase, producing soluble β-APP fragments (sAPPβ) and C-terminal β fragment (CTFβ, C99), and C99 is further cleaved by γ-secretase, generating APP intracellular domain (AICD) and Aβ. The non-amyloidogenic pathway is an innate way to prevent the generation of Aβ, as APP is firstly recognized by α-secretase within Aβ domain, generating soluble α-APP fragments (sAPPα) and C-terminal fragment α (CTFα, C83), C83 is then cleaved by γ-secretase, producing non-toxic P3 and AICD fragments.