Abstract
Collagenases are critical reagents determining yield and quality of isolated human pancreatic islets and may affect islet transplantation outcome. Some islet transplantation centers have compared 2 or more collagenase blends; however, the results regarding differences in quantity and quality of islets are conflicting. Thus, for the first time, a mixed treatment comparison (MTC) meta-analysis was carried out to compile data about the effect of different collagenases used for human pancreas digestion on islet yield, purity, viability and stimulation index (SI). Pubmed, Embase and Cochrane libraries were searched. Of 755 articles retrieved, a total of 15 articles fulfilled the eligibility criteria and were included in the MTC meta-analysis. Our results revealed that Vitacyte and Liberase MTF were associated with a small increase in islet yield (islet equivalent number/g pancreas) when compared with Sevac enzyme [standardized mean difference (95% credible interval – CrI) = −2.19 (−4.25 to −0.21) and −2.28 (−4.49 to −0.23), respectively]. However, all other enzyme comparisons did not show any significant difference regarding islet yield. Purity and viability percentages were not significantly different among any of the analyzed digestion enzymes. Interestingly, Vitacyte and Serva NB1 were associated with increased SI when compared with Liberase MTF enzyme [unstandardized weighted mean difference (95% CrI) = −1.69 (−2.87 to −0.51) and −1.07 (−1.79 to −0.39), respectively]. In conclusion, our MTC meta-analysis suggests that the digestion enzymes currently being used for islet isolation works with similar efficiency regarding islet yield, purity and viability; however, Vitacyte and Serva NB1 enzymes seem to be associated with an improved SI as compared with Liberase MTF.
Keywords: digestion collagenase, enzyme, human islet isolation, meta-analysis, mixed treatment comparison
Abbreviations
- BMI
body mass index
- CrIs
credible intervals
- cGMP
current good manufacturing practice
- CIT
cold ischemia time
- FE
fixed effect
- GRADE
grading of recommendations assessment, development and evaluation
- IEQ
islet equivalent number
- MTC
mixed treatment comparison
- NB
neutral protease
- WMD
weighted mean difference
- RE
random effect
- SI
stimulation index
- SMD
standardized mean difference
- T1DM
type 1 diabetes mellitus
Introduction
Human islet transplantation has become a promising treatment for type 1 diabetes mellitus (T1DM) since several centers worldwide have replicated the isolation and transplantation procedures defined by the Edmonton Protocol.1,2 The Collaborative Islet Transplant Registry (CITR) has recently published data from last decade, showing improvement in efficacy and safety outcomes of islet transplantation.3 Both number of islet infusions and adverse events per recipient dropped significantly in 2007–2010 as compared to 1999–2006.3 However, some centers were consistently more successful than others,4 suggesting that post isolation islet quality and, consequently, transplantation success are highly sensitive to the expertise and experience of the centers performing the procedure.
Tissue dissociation enzymes are critical reagents determining yield and quality of isolated pancreatic islets and may affect clinical outcomes.5 The enzyme Liberase HI (Roche, Indianapolis, USA) has been widely used in human islet isolation, and was considered the enzyme of choice.5,6 However, in 2007, serious concerns regarding the utilization of this enzyme were raised up after the discovery that its manufacturing process involved bovine brain-derived raw material, which has the potential to transmit prion-associated diseases.7 Since then, efforts have been made to replace this enzyme, such as purification of collagenase and protease enzyme blend components as well as characterization of composition and digestion efficacy of new enzymes.8,9
Current state-of-the-art digestion enzyme blends included collagenase NB1 (Serva, Heidelberg, Germany), a mammalian tissue-free version of Liberase (Roche, Indianapolis, USA), and the new blend Vitacyte (Vitacyte, Indianapolis, USA).4,10-12 Although, some centers have compared 2 or more enzymes, the results regarding differences in islet yield and viability are still conflicting.5,6,8,12-14
Liberase HI and Serva NB1 have been the most widely investigated enzymes for human islet isolation.4 Nevertheless, even today, there is no standardization of collagenase digestion in human islet isolation between centers, and this might account, in part, for the high variability in islet isolation results.11,15 Moreover, all digestion enzymes have never been directly compared with respect to islet isolation outcomes in the same study. Given that evidence-based decision-making requires comparison of all relevant competing interventions, network meta-analysis might provide useful evidence for judiciously selecting the best enzyme. In this context, mixed treatment comparison (MTC) meta-analysis, a special case of network meta-analysis, could compare all digestion enzymes using a single model. As a result, pairwise comparisons are made by combining direct and indirect evidence or by using direct or indirect evidence when only one of them is available, thereby synthesising a greater share of the available evidence than a traditional meta-analysis.16,17 Therefore, in order to define the most appropriate enzyme to be used in human islet isolation, we performed a MTC meta-analysis of studies that reported human islet isolation and evaluated the effect of different pancreas digestion enzymes regarding to islet equivalent number [(IEQ)/g pancreas], viability, purity and glucose-stimulated insulin release [stimulated index (SI)] of the islet product. Analyzed enzymes were as follows: Liberase HI, Serva NB1, Vitacyte, Liberase MTF, Collagenase P (Boehringer Mannheim, Indianapolis, USA), Sevac (Crescent Chemical, Hauppauge, USA), Sigma V (Sigma, St. Louis, USA), Recombinant (Roche, Penzberg, Germany) and Collagenase Custom (Roche, Indianapolis, USA).
Results
Literature search results and characteristics of the eligible studies
Figure 1 is a flow diagram illustrating the strategy used to identify and select studies for inclusion in this systematic review and MTC meta-analysis. A total of 755 potentially relevant citations were retrieved by searching the electronic databases, and 699 of them were excluded during the review of titles and abstracts. Fifty-6 articles, therefore, appeared to be eligible at this point and had their full texts evaluated. However, after critical reading of full texts, another 41 studies were excluded because of ineligible study designs, duplicated results, absence of comparison of at least 2 types of collagenases, no outcome of interest analyzed, islet isolation from non-human pancreas and autologous islet transplantation. A total of 15 articles fulfilled the eligibility criteria and were included in the MTC meta-analysis.
Figure 1.
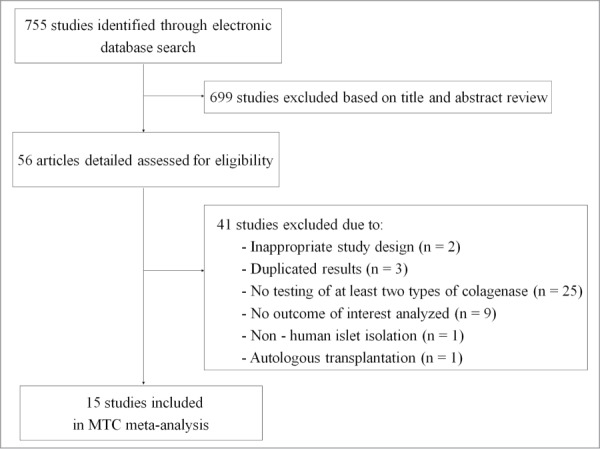
Flowchart illustrating the search strategy used in the systematic review and mixed treatment comparison meta-analysis.
Supplementary Table 1 summarizes the characteristics of the selected studies. Among them, 2 compared more than 2 enzymes:8,13 one compared Liberase HI, Serva NB1, and Collagenase P,8 and the other compared Liberase HI, Serva NB1, Vitacyte and Liberase MTF.13 Five studies compared Liberase HI vs. Serva NB1,5,10,11,14,18 2 compared Serva NB1 vs. Vitacyte,19,20 one compared Serva NB1 vs. Sigma V,15 and one compared Serva NB1 vs. Liberase MTF.12 Four were conducted comparing Liberase HI with other different enzymes (Collagenase P, Sevac, Recombinant and Collagenase Custom).6,21-23 Supplementary Table 2 depicts clinical characteristics of the pancreatic donors.
Evidence network
Figure 2 shows the evidence network of enzyme comparisons. From the evidence network is possible to observe that some pairwise comparisons have only direct evidence that comes from head-to-head studies (Custom vs. Liberase HI, for example), some have only indirect evidence (for example, Custom vs. Sevac evidence comes indirectly from the trials comparing Custom vs. Liberase HI and Sevac vs. Liberase HI) and other pairs have both direct and indirect evidence (Fig. 2A). Nine enzymes were compared for the outcome IEQ/g pancreas (Fig. 2A), including 15 studies and totalizing 1211 analyzed pancreases. Seven enzymes were compared for viability (Fig. 2B) and derived from 8 studies including 451 pancreases. Seven enzymes were also compared for purity (Fig. 2C), derived from 9 studies including 794 pancreases. Lastly, 7 enzymes were compared for SI (Fig. 2D), derived from 11 studies including 639 pancreases.
Figure 2.
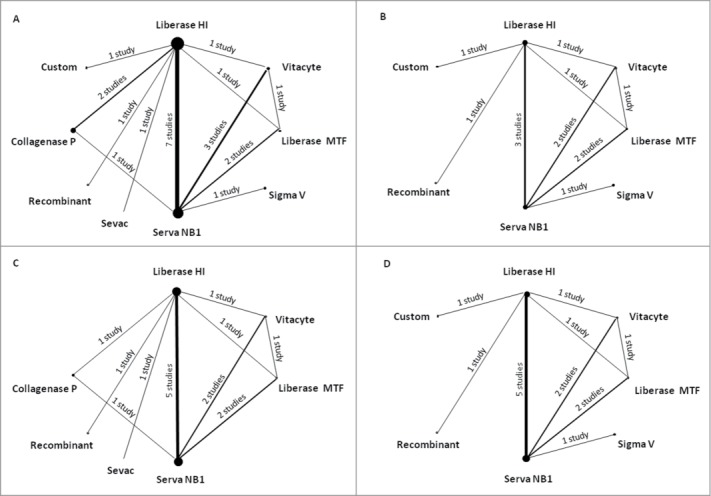
Network of eligible comparisons for the mixed-treatment comparison meta-analysis. (A) islet equivalent number (IEQ)/g pancreas; (B) Viability; (C) Purity; (D) stimulation index (SI). Lines connect the enzymes that have been studied in head-to-head (direct) comparisons. The width of the lines represents the cumulative number of studies for each comparison, and the size of each node is proportional to the sample size.
Islet equivalent number (IEQ)/g pancreas and viability
Figure 3A summarizes the results of the MTC meta-analysis of the effect of different digestion enzymes on the standardized mean difference (SMD) of the IEQ/g pancreas and on the unstandardized weighted mean difference (WMD) of the islet viability. Vitacyte and Liberase MTF enzymes were associated with significantly better results on IEQ/g pancreas as compared with the Sevac enzyme [−2.19 (−4.25 to −0.21) and −2.28 (−4.49 to −0.23), respectively]. However, Vitacyte and Liberase MTF showed no significant difference on this outcome when compared with the other enzymes (Liberase HI, Serva NB1, Collagenase P, Sigma V, and Collagenase Custom). Moreover, these other enzymes also did not show differences when compared with each other. Our results revealed that percentages of viability were not significantly different when using any of the analyzed digestion enzymes. It is noteworthy that we repeated these MTC meta-analyses including only Liberase HI, Serva NB1, Vitacyte and Liberase MTF enzymes. Importantly, the results obtained were similar for those of IEQ/g pancreas and viability MTC meta-analyzes when all enzymes were included (Supplementary Fig. S1). Moreover, repeating the all enzyme-meta-analysis excluding only Liberase HI, which is not commercially available anymore, the data did not change significantly (Supplementary Fig. S2).
Figure 3.
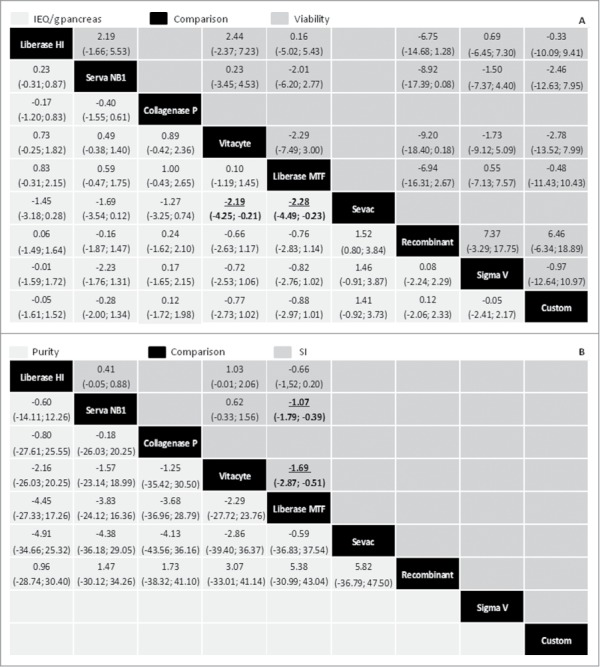
Mixed-treatment comparison (MTC) meta-analysis of the effect of different digestion enzymes on changes of islet equivalent number (IEQ)/g pancreas, viability, islet purity and stimulation index (SI). (A) MTC of the effect of different enzymes on the median of the standardized mean difference (SMD) of the IEQ/g pancreas and on the median of the unstandardized weighted mean difference (WMD) of viability. Results are SMD and WMD [(95% credible interval (CrI)] in the column-defining enzyme compared with the row-defining enzyme. For IEQ/g pancreas a negative value favors the column-defining enzyme, and for viability a negative value favors the row-defining enzyme. (B) MTC of the effects of different enzymes on the median of the unstandardized WMD of islet purity and SI. Results are WMD (95% CrI) in the column-defining enzyme compared with the row-defining enzyme. For purity, a negative value favors the column-defining enzyme, and for SI, a negative value favors the row-defining enzyme. Significant results are in bold and underlined.
Figure 4 shows the estimated probability that each enzyme is ranked first, second, third and so on as being the most effective enzyme in generating better results on the analyzed outcomes. For the outcome IEQ/g pancreas, Liberase MTF was the enzyme that had the greatest probability of being the best enzyme (42%), followed by Vitacyte (29%) (Fig. 4A). The other enzymes showed similar probabilities of being ranked the first best enzyme. For viability outcome, the Vitacyte enzyme showed a 38% probability of being the most effective enzyme in generating higher islet viability, followed by Collagenase Custom (24%) and Serva NB1 (20%) enzymes (Fig. 4B).
Figure 4.
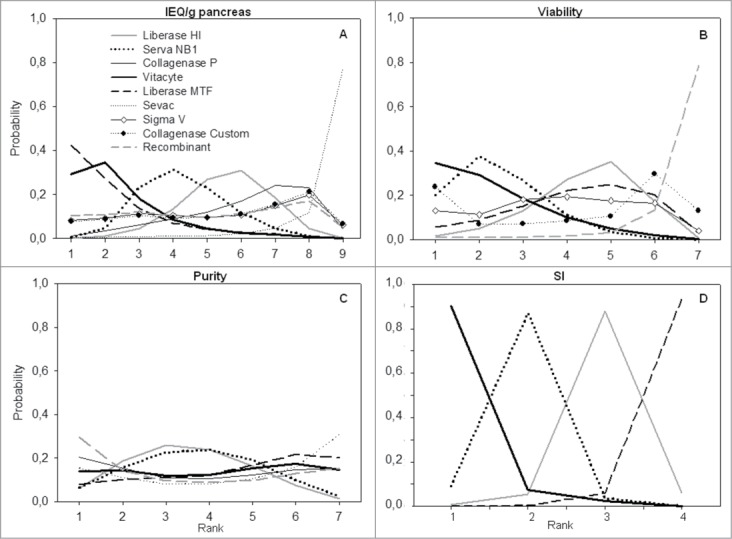
Plots for ranking probability of different enzymes in generating better results on the analyzed outcomes. Ranking = probability of being the best enzyme, of being the second best, the third best and so on, among all enzymes. (A) Rank for islet equivalent number (IEQ)/g pancreas; (B) Rank for islet viability; (C) Rank for the islet purity; and (D) rank for stimulation index (SI).
Purity and stimulation index (SI)
Figure 3B summarizes the results of the MTC meta-analysis of the effect of different enzymes on the unstandardized weighted mean difference (WMD) of purity and SI of the islet product. Our results revealed that percentages of purity were not significantly different when using any of the analyzed digestion enzymes. In the same way as performed for IEQ/g pancreas and viability outcomes, we also repeated the meta-analysis including only Liberase HI, Serva NB1, Vitacyte and Liberase MTF enzymes, with the results also indicating that these 4 enzymes are associated with similar percentages of purity (Supplementary Fig. S1). When repeating the all enzyme-meta-analysis excluding Liberase HI, the data regarding purity also did not change significantly (Supplementary Fig. S2).
For the SI meta-analysis, we only compared Liberase HI, Serva NB1, Vitacyte and Liberase MTF enzymes because of the high variability observed on SI values obtained for islets isolated using the other enzymes. Interestingly, Vitacyte and Serva NB1 enzymes were associated with significantly increased SI as compared with Liberase MTF enzyme [−1.69 (−2.87 to −0.51) and −1.07 (−1.79 to −0.39), respectively, Figure 3]. After excluding the Liberase HI from this analysis, the data also did not change significantly (Supplementary Fig. S1).
When evaluating the estimated probability of each enzyme being the best effective enzyme in generating better purity (Fig. 4C), the Recombinant enzyme showed the greatest probability of being the best enzyme (30%), followed by Collagenase P (20%), Vitacyte and Sevac (15%), Liberase MTF (8%), and Serva NB1 and Liberase HI (6%). Regarding the SI outcome, the Vitacyte enzyme showed a 90% probability of being the most effective enzyme, followed by Serva NB1 (9%) (Fig. 4D).
Data quality and inconsistence checking
We assessed the quality of each individual study included in the meta-analyses using GRADE (The Grading of Recommendation Assessment, Development and Evaluation) recommendations.24 For each outcome (IEQ/g pancreas, islet viability, purity and SI), studies were evaluated for risk of bias, imprecision, inconsistency, indirectness, magnitude of the treatment effect and presence of a dose-response gradient. The quality of the evidence was then classified as high, moderate, low or very low. Studies included in our MTC meta-analyses are not-blinded observational studies. Therefore, using the GRADE recommendations, the evidence was classified as low to very low quality for the 4 outcomes.
One key assumption of MTC meta-analysis models is the consistency between direct and indirect evidence, that is, if the information of both sources of evidence are similar enough in order to be combined. Therefore, the interpretation of our results should rely on this assumption.17,25 Thus, it is important to point out that our results showed that there is no significant inconsistency between direct and indirect evidences for the outcomes IEQ/g pancreas, islet viability, purity and SI (see Supplementary Table 3); thus, our MTC meta-analysis is statistically adequate and presents reliable data.
Discussion
In the present MTC meta-analysis, the most common collagenases used for pancreas digestion were similar for islet yield, islet purity, and islet viability. The only exception was a small and not clinically relevant increment in the islet yield when Vitacyte and Liberase MTF enzymes were used as compared with Sevac. In addition, Vitacyte and Serva NB1 enzymes were associated with higher SI values as compared to Liberase MTF enzyme. Importantly, when performing MTC meta-analyses including only Liberase HI, Liberase MTF, Serva NB1 and Vitacyte enzymes, the results were similar to the results obtained for the complete meta-analyses. Moreover, our data showed no significant inconsistency between direct and indirect evidences for all outcomes, indicating that our MTC meta-analysis presents reliable data. Islet yield (IEQ/g pancreas), viability and purity were chosen as outcomes for our meta-analysis since they are validated islet batch product release criteria for human islet transplantation.26 SI was also chosen as an outcome for this study since it is an important parameter of islet function.
The original islet isolation protocol currently being used at most centers was first described by Ricordi et al.27 in 1988 and, since then, each center has refined this protocol. Centers have improved their own isolation protocols by developing new digestion enzyme combinations, by modifying the media and procedures, and by adding various reagents to the culture media.28 However, even though a human pancreas contains an estimated one to 2 million islets,29 it is still difficult for islet processing laboratories to consistently obtain sufficient quantities of intact islets from different donors.29,30 Scientists performing human islet isolation face several challenges to get a sufficient yield of islet for clinical transplantation, which is typically achieved only in fewer than 45% of human donor pancreas isolations.31 Assuming that the team is experienced in islet isolation, the 2 primary factors that influence islet yield and quality are donor variables and the tissue digestion enzyme quality.32 In this scenario, the standardization of all procedures used during human islet isolation is an essential requisite for the reproducible production of sufficient numbers of good-quality islets and, consequently, for a successful clinical transplantation.28,33
The critical procedure of islet isolation is a mechanically enhanced enzymatic digestion of the pancreas, which allows dissociation and freeing of the islets from the acinar tissue.34,35 Collagenase plays a crucial role in dissociating the pancreas during enzymatic digestion phase,8 but the heterogeneity of enzyme preparations has hindered the standardization of the collagenase digestion protocol in human islet isolation, and continue to hamper a process that is inherently difficult to control.15,29 Some studies have attempted to identify the most suitable digestion enzyme to be used in islet isolation.5,8,13,14,19,20 However, taking into account that these studies analyzed different enzymes, and used different enzyme batch or different isolation protocols, presently, it is difficult to conclude which, among the available digestion enzymes, best suits the islet isolation procedure. Thus, making many laboratories base the selection on personal experience.
The Liberase HI was introduced in 1994 as the first dissociation enzyme blend specially developed to isolate human islets. This low-endotoxin product contains purified collagenase from C. histolyticum, class I and class II isoforms, and a thermostable purified bacterial neutral protease (NP).6,20 Class I and II isoforms are identified by differences in their substrate specificity and work synergistically to degrade collagen.33 Neutral protease is thought to further enhance the degradation of all major components of the extracellular matrix, also playing an important role in enzymatic digestion.32,33 The development of Liberase HI dramatically improved human islet isolation, consistently yielding large number of islets without compromising their functional viability, and has become the preferred blend for clinical islet isolation.6,29 Unfortunately, in 2007, following recommendations from the Food and Drug Administration (FDA), this enzyme blend was withdrawn from the market due to the potential, although low, risk of prion disease transmission.7,10 After the Liberase HI became unavailable for clinical use, clinical islet transplant centers were forced to identify a substitute enzyme that consistently produced sufficient number of good-quality islets for transplantation.
The enzyme blend that then became available and is now used by many centers is a combination of a collagenase NB1 and a NP manufactured by Serva Electrophoresis.10,36 Collagenase NB1 and NP are reconstituted separately and mixed before use to minimize degradation of the collagenase by NP. Consequently, the amounts of collagenase and NP can be adjusted independently to match the size of pancreas being processed.10,36 According to the certificate of analysis, the biochemical activity of Serva NB1 is similar to Liberase HI, but its parameters for optimal use are different and many experienced centers have had inconsistent results using this product.10,36 Interestingly, recent data from CITR show improvement in primary efficacy and safety outcomes of islet transplantation in recipients who received transplants in 2007–2010 compared with those in 1999–2006, with fewer islet infusions and adverse events per recipient.3 Although these dates coincide with the switch in the use of Liberase HI enzyme to Serva NB1 enzyme, it is not possible to conclude that the improvement of the islet transplantation results in more recent years is only due to the utilization of Serva NB1 blend, since no randomized clinical trials were performed specifically to compare these 2 enzymes.
An analysis of more than 400 human islet isolations demonstrated that the efficacy of an enzyme blend is extremely important for a successful isolation process and posttransplant function after human islet allotransplantation.37 Consequently, the selection of an appropriate enzyme blend is a critical step in the process of islet isolation. Thus, aiming at improvement of islet isolation, other state-of-the-art enzyme blends were recently developed: Liberase MTF and Vitacyte. Similarly to Serva NB1, these 2 new enzymes are also provided in 2 separate vials, one of them containing the collagenase and the other containing the non-collagenolytic enzyme.4.10-12 At the present time, Serva NB1, Liberase MTF, and Vitacyte seem to perform with similar success, but head-to-head comparisons are pending to determine the optimal enzyme blend that maximizes human islet yield and quality.4 As already commented, some centers have compared 2 or more enzymes and were included in our meta-analysis, but individual results regarding differences in islet yield and viability are conflicting.5,8,13,14,19,20
In this scenario, our MTC meta-analysis combined direct and indirect evidence; allowing comparisons among enzymes even when head-to-head studies were not available. Combining results from direct and indirect evidences in a MTC model might yield a more refined and precise estimate of the interventions directly compared and a broader inference to the population sampled because it links and maximizes existing information within the network of treatment comparisons. Moreover, a major advantage of this approach is that the method naturally leads to a decision framework that supports decision making.16,17 Such a fact might help researches in selecting the most appropriate enzyme for islet isolation. In this context, our results did not indicate any significant difference between the enzymes most commonly used regarding IEQ/g pancreas, islet purity and viability. Nevertheless, Vitacyte and Serva NB1 seem to be associated with an improved SI as compared to Liberase MTF enzyme. It is worth noting that when we evaluated the ranking of the best enzyme for IEQ/g pancreas, Liberase MTF and Vitacyte demonstrated a slightly superiority when compared to the other enzymes. Furthermore, regarding the most used enzymes nowadays, Vitacyte and Serva NB1 also seems to be superior when analyzing viability and SI.
Recently, Balamurugan et al.38 evaluated 8 different enzyme combinations in an attempt to improve islet yield. The enzyme combinations consisted of purified, intact or truncated Class I and Class II collagenases from C. histolyticum (Ch) and a NP from Bacillus thermoproteolyticus rokko (thermolysin) or from Ch. A new enzyme mixture composed of the Vitacyte blend and a NP from Ch. was able to recover the highest islet yield while retaining islet quality.38 Probably, future comparisons will be made analyzing this new enzyme mixture.
The present study has some limitations. First, most of the articles included are observational studies rather than randomized clinical trials. Second, the general quality of these studies was considered low,24 raising the possibility of bias. Third, for some enzyme comparisons, only a few studies were available to be included in this study. However, when we repeated the IEQ/g pancreas, islet viability and purity MTC analyses including only Liberase MTF, Liberase HI, Serva NB1 and Vitacyte our results did not significantly change (Supplementary Fig. S1). Taking into account that Liberase HI is not used nowadays, we repeated the complete meta-analyses excluding Liberase HI (Supplementary Fig. S2), and this also did not significantly change the results. Fourth, heterogeneity is potentially a significant problem when interpreting the results of any meta-analysis, and our meta-analysis showed significant inter-study heterogeneity in almost all analyses. The heterogeneity that we observed could originate from the improvement of islet isolation over the years, as well as differences in donor selection, enzyme batches, isolation procedures, culture medium and other reagents used for promoting islet survival. Besides that, it could also raise from differences in expertise of the isolation teams,28,33,39-42 and from the fact that techniques used for evaluation of SI and viability can be slightly different between studies. Regarding insulin secretion, studies used different glucose incubation time and different high and low glucose concentrations. Concerning viability, all included studies used fluorescent dyes to mark viable and dead cells, but these dyes not necessarily were the same. Without detailed information on these variables we cannot exclude the possibility that the heterogeneity observed might reduce our power to detect true differences between enzymes. Furthermore, all member of an islet isolation team knows that the efficacy of an enzyme depends on its batch. Unfortunately, due to the lack of data, we were not able to control for collagenase batches. However, we believe that our results are still robust since we applied a Bayesian model to explore the effects of indirect evidence between studies, which is thought to be the most appropriate method for MTC meta-analysis.16,43
In summary, improvement of islet isolations and standardization of these procedures are important prerequisites for the success of clinical islet transplantation. Thus, although this MTC meta-analysis has some limitations, this is the first study that provides a useful and complete picture of the comparison between the enzymes used in islet isolation. Our MTC meta-analysis suggests that analyzed enzymes works with similar efficiency regarding to islet yield, purity and viability, but Vitacyte and NB1 enzymes seem to work better regarding SI. Moreover, ranking of efficacy evaluations suggest that Liberase MTF and Vitacyte blends have a slightly superiority regarding IEQ/g pancreas outcome compared to the other enzymes. Among the most used enzymes nowadays, Vitacyte and Serva NB1 seem to be superior when analyzing SI and viability outcomes. Thus, as affirmed by McCall and Shapiro4 and based in our ranking of efficacy data, we believe that, at the current time, Vitacyte, Serva NB1, and possible Liberase MTF enzymes seem to perform with similar success, and should be the enzymes of choice for new islet isolation centers. Nevertheless, new head-to-head enzyme comparisons are urgently needed to more optimally define the best enzyme for the standardization of the islet isolation procedure.
Methods
Search strategy and study selection
This study was designed and described in accordance with current guidelines.44,45 To identify studies that reported human islet isolation and compared different enzymes for pancreas digestion, we performed an electronic literature search in Medline, Embase and Cochrane libraries, without date restriction, using the following medical subject readings (MeSH): “pancreas islet transplantation” OR “pancreas islet and collagenase” OR “islet isolation” AND “clostridiopeptidase” OR “collagenase” OR “Serva” OR “Liberase” OR “Sigma V” OR “Vitacyte” OR “Liberase MTF” OR “NB1 Premium Grade” OR “CIzyme collagenase HA” OR “Liberase HI” OR “Clostridium histolyticum.” The search was limited to human and English or Spanish language papers and was completed on August 22, 2013.
Two investigators (J.R. and R.C.) independently reviewed the titles and abstracts of all retrieved articles in order to evaluate whether the studies were eligible for inclusion in the systematic review. Disagreements were resolved by discussion between them and when necessary a third reviewer (DC) was consulted. When abstracts did not provide enough information regarding the inclusion and exclusion criteria, the full text of the article was retrieved for evaluation. Studies were considered eligible for inclusion if they fulfilled the following criteria: 1) the study should have been performed in human pancreatic islet isolation; 2) it should have tested at least 2 types of collagenases; and 3) it should have analyzed one or more of the outcomes of interest: IEQ/g pancreas, islet viability, purity of the islet product and SI. If data were duplicated and had been published more than once, the most complete study was chosen. Articles that did not fulfill the eligibility criteria described above were excluded from the analysis.
Data extraction and quality analysis
Data were independently extracted by 2 investigators (J.R. and R.C) using a standardized abstraction form and a consensus was sought in all extracted items. When consensus could not be reached, differences in data extraction were resolved by a third reviewer (D.C or C.B.L.) and by referencing the original publication. The information extracted from each individual study was as follows: author's first name, publication year, name of collagenases used, number of subjects in each group, donor's characteristics (age, gender, weight, BMI, and cause of death), characteristics of the pancreas (weight, CIT, and digestion time), and data about the outcomes of interest previously described.
Two investigators (J.R. and R.C) independently assessed the quality of each eligible study using GRADE recommendation.25 GRADE classifies quality of evidence into 4 categories: high, moderate, low or very low. The quality assessment includes factors such as the study design (risk of bias), imprecision, inconsistency, indirectness, and publication bias. Some factors, which may lead to rating up quality, were including – magnitude of treatment effect and the presence of a dose–response gradient.25
Statistical analyses
Outcomes of interest were IEQ/g pancreas, islet viability, purity and SI. The effects of each digestion enzyme on the outcome were analyzed using a MTC meta-analysis, conducted by using Markov-Chain Monte Carlo simulations and fitted in the software WinBUGS version 1.4.3 (Medical Research Council Biostatistics Unit, Cambridge, UK). The MTC meta-analysis is a generalization of traditional pairwise meta-analysis that allows all evidence from multiple treatments to be taken into account simultaneously in a single model, combining direct and indirect evidence.16,17
For this MTC meta-analysis, the Liberase HI enzyme was considered the baseline enzyme and both fixed effect (FE) and random effect (RE) models were fitted for each outcome. The choice between FE or RE models was made comparing the competing models using the deviance information criteria (DIC). For each model, goodness-of-fit to the data was evaluated using residual deviance.46
Therefore, the results are shown for RE models conducted using Markov chains. Each chain used 300,000 iterations with a burn-in of 50,000 and a tinning interval of 40. Vague prior distributions were used for all models. Due to the high values found for the variable IEQ/g pancreas, the results for this outcome are summarized as median of the SMD calculated by the Hedges method.42,45 For viability, purity and SI, the results are shown as median of the unstandardized WMD. For all outcomes, 95% credible intervals (CrIs, the Bayesian equivalents of CI) are also shown.16
One particularly useful characteristic of MTC models is the possibility to calculate the expected ranking of efficacy for all treatments based on the posterior probabilities of all treatment rankings (i.e., probability of being the best, probability of second best, and so on).16,17,47 Thus, we calculated the probability of each digestion enzyme to be the most effective (first-best) enzyme, the second-best, and so on, for our outcomes, and presented the results in Figure 4.
When direct and indirect evidences are combined for a particular pairwise comparison, it is important to check if there is no discrepancy between these evidences. Therefore, this consistency assumption should be accounted for.17 If consistency between direct and indirect evidences is observed, the data is statistically reliable. In this study, the consistency assumption was checked using posterior plots and the Bayesian P-values produced by the node-splitting method proposed by Dias et al.25 In this approach, each pairwise comparison in a closed loop of the network has its direct and indirect components compared. The evidence of a pair was considered inconsistent if the P value was smaller than the significance level (α = 0.05) adjusted for multiple comparisons.25
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by grants from Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Fundo de Incentivo à Pesquisa e Eventos (FIPE) at Hospital de Clínicas de Porto Alegre. C.B.L. and DC are recipients of scholarships from CNPq.
Supplemental Materials
Supplemental data for this article can be accessed on the publisher's website.
References
- 1. Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000; 343:230-8; PMID:10911004; http://dx.doi.org/ 10.1056/NEJM200007273430401 [DOI] [PubMed] [Google Scholar]
- 2. CITR Research Group . 2007 update on allogeneic islet transplantation from the Collaborative Islet Transplant Registry (CITR). Cell Transplant 2009; 18:753-67; PMID:19796497; http://dx.doi.org/ 10.3727/096368909X470874 [DOI] [PubMed] [Google Scholar]
- 3. Barton FB, Rickels MR, Alejandro R, Hering BJ, Wease S, Naziruddin B, Oberholzer J, Odorico JS, Garfinkel MR, Levy M, et al. . Improvement in outcomes of clinical islet transplantation: 1999–2010. Diabetes Care 2012; 35:1436-45; PMID:22723582; http://dx.doi.org/ 10.2337/dc12-0063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McCall M, Shapiro AM. Update on islet transplantation. Cold Spring Harb Persp Med 2012; 2:a007823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Misawa R, Ricordi C, Miki A, Barker S, Molano RD, Khan A, Miyagawa S, Inverardi L, Alejandro R, Pileggi A, et al. . Evaluation of viable beta-cell mass is useful for selecting collagenase for human islet isolation: comparison of collagenase NB1 and liberase HI. Cell Transplant 2012; 21:39-47; PMID:21929867; http://dx.doi.org/ 10.3727/096368911X582732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Linetsky E, Bottino R, Lehmann R, Alejandro R, Inverardi L, Ricordi C. Improved human islet isolation using a new enzyme blend, liberase. Diabetes 1997; 46:1120-3; PMID:9200645; http://dx.doi.org/ 10.2337/diab.46.7.1120 [DOI] [PubMed] [Google Scholar]
- 7. Alejandro R, Barton FB, Hering BJ, Wease S. 2008 Update from the Collaborative Islet Transplant Registry. Transplantation 2008; 86:1783-8; PMID:19104422; http://dx.doi.org/ 10.1097/TP.0b013e3181913f6a [DOI] [PubMed] [Google Scholar]
- 8. Sabek OM, Cowan P, Fraga DW, Gaber AO. The effect of isolation methods and the use of different enzymes on islet yield and in vivo function. Cell Transplant 2008; 17:785-92; PMID:19044205; http://dx.doi.org/ 10.3727/096368908786516747 [DOI] [PubMed] [Google Scholar]
- 9. Bertuzzi F, Cainarca S, Marzorati S, Bachi A, Antonioli B, Nano R, Verzaro R, Ricordi C. Collagenase isoforms for pancreas digestion. Cell Transplant 2009; 18:203-6; PMID:19499708; http://dx.doi.org/ 10.3727/096368909788341270 [DOI] [PubMed] [Google Scholar]
- 10. Szot GL, Lee MR, Tavakol MM, Lang J, Dekovic F, Kerlan RK, Stock PG, Posselt AM. Successful clinical islet isolation using a GMP-manufactured collagenase and neutral protease. Transplantation 2009; 88:753-6; PMID:19920770; http://dx.doi.org/ 10.1097/TP.0b013e3181b443ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brandhorst H, Friberg A, Nilsson B, Andersson HH, Felldin M, Foss A, Salmela K, Tibell A, Tufveson G, Korsgren O, et al. . Large-scale comparison of Liberase HI and collagenase NB1 utilized for human islet isolation. Cell Transplant 2010; 19:3-8; PMID:19818208; http://dx.doi.org/ 10.3727/096368909X477507 [DOI] [PubMed] [Google Scholar]
- 12. O’Gorman D, Kin T, Imes S, Pawlick R, Senior P, Shapiro AM. Comparison of human islet isolation outcomes using a new mammalian tissue-free enzyme versus collagenase NB-1. Transplantation 2010; 90:255-9; http://dx.doi.org/ 10.1097/TP.0b013e3181e117ce [DOI] [PubMed] [Google Scholar]
- 13. Shimoda M, Noguchi H, Naziruddin B, Fujita Y, Chujo D, Takita M, Peng H, Tamura Y, Olsen GS, Sugimoto K, et al. . Assessment of human islet isolation with four different collagenases. Transplant Proc 2010; 42:2049-51; PMID:20692404; http://dx.doi.org/ 10.1016/j.transproceed.2010.05.093 [DOI] [PubMed] [Google Scholar]
- 14. Iglesias I, Valiente L, Shiang KD, Ichii H, Kandeel F, Al-Abdullah IH. The Effects of Digestion Enzymes on Islet Viability and Cellular Composition. Cell Transplant 2012; 21:649-55; PMID:22236690; http://dx.doi.org/ 10.3727/096368911X623826 [DOI] [PubMed] [Google Scholar]
- 15. Wang Y, Paushter D, Wang S, Barbaro B, Harvat T, Danielson K, Kinzer K, Zhang L, Qi M, Oberholzer J. Highly purified versus filtered crude collagenase: comparable human islet isolation outcomes. Cell Transplant 2011; 20:1817-25; PMID:21396158; http://dx.doi.org/ 10.3727/096368911X564994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 2004; 23:3105-24; PMID:15449338; http://dx.doi.org/ 10.1002/sim.1875 [DOI] [PubMed] [Google Scholar]
- 17. Jansen JP, Fleurence R, Devine B, Itzler R, Barrett A, Hawkins N, Lee K, Boersma C, Annemans L, Cappelleri JC. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR task force on indirect treatment comparisons good research practices: part 1. Value in Health 2011; 14:417-28; PMID:21669366; http://dx.doi.org/ 10.1016/j.jval.2011.04.002 [DOI] [PubMed] [Google Scholar]
- 18. Bucher P, Mathe Z, Morel P, Bosco D, Andres A, Kurfuest M, Friedrich O, Raemsch-Guenther N, Buhler LH, Berney T. Assessment of a novel two-component enzyme preparation for human islet isolation and transplantation. Transplantation 2005; 79:91-7; PMID:15714175; http://dx.doi.org/ 10.1097/01.TP.0000147344.73915.C8 [DOI] [PubMed] [Google Scholar]
- 19. Caballero-Corbalan J, Friberg AS, Brandhorst H, Nilsson B, Andersson HH, Felldin M, Foss A, Salmela K, Tibell A, Tufveson G, et al. . Vitacyte collagenase HA: a novel enzyme blend for efficient human islet isolation. Transplantation 2009; 88:1400-2; PMID:20029339; http://dx.doi.org/ 10.1097/TP.0b013e3181bd1441 [DOI] [PubMed] [Google Scholar]
- 20. Balamurugan AN, Breite AG, Anazawa T, Loganathan G, Wilhelm JJ, Papas KK, Dwulet FE, McCarthy RC, Hering BJ. Successful human islet isolation and transplantation indicating the importance of class 1 collagenase and collagen degradation activity assay. Transplantation 2010; 89:954-61; PMID:20300051; http://dx.doi.org/ 10.1097/TP.0b013e3181d21e9a [DOI] [PubMed] [Google Scholar]
- 21. O’Gorman D, Kin T, McGhee-Wilson D, Shapiro AM, Lakey JR. Multi-lot analysis of custom collagenase enzyme blend in human islet isolations. Transplant Proc 2005; 37:3417-9; http://dx.doi.org/ 10.1016/j.transproceed.2005.09.139 [DOI] [PubMed] [Google Scholar]
- 22. Olack BJ, Swanson CJ, Howard TK, Mohanakumar T. Improved method for the isolation and purification of human islets of langerhans using Liberase enzyme blend. Hum Immunol 1999; 60:1303-9; PMID:10626746; http://dx.doi.org/ 10.1016/S0198-8859(99)00118-4 [DOI] [PubMed] [Google Scholar]
- 23. Brandhorst H, Brandhorst D, Hesse F, Ambrosius D, Brendel M, Kawakami Y, Bretzel RG. Successful human islet isolation utilizing recombinant collagenase. Diabetes 2003; 52:1143-6; PMID:12716744; http://dx.doi.org/ 10.2337/diabetes.52.5.1143 [DOI] [PubMed] [Google Scholar]
- 24. Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, et al. . GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011; 64:401-6; PMID:21208779; http://dx.doi.org/ 10.1016/j.jclinepi.2010.07.015 [DOI] [PubMed] [Google Scholar]
- 25. Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med 2010; 29:932-44; PMID:20213715; http://dx.doi.org/ 10.1002/sim.3767 [DOI] [PubMed] [Google Scholar]
- 26. Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, et al. . International trial of the Edmonton protocol for islet transplantation. N Engl J Med 2006; 355:1318-30; PMID:17005949; http://dx.doi.org/ 10.1056/NEJMoa061267 [DOI] [PubMed] [Google Scholar]
- 27. Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes 1988; 37:413-20; PMID:3288530; http://dx.doi.org/ 10.2337/diab.37.4.413 [DOI] [PubMed] [Google Scholar]
- 28. Yamamoto T, Horiguchi A, Ito M, Nagata H, Ichii H, Ricordi C, Miyakawa S. Quality control for clinical islet transplantation: organ procurement and preservation, the islet processing facility, isolation, and potency tests. J Hepatobiliary Pancreat Surg 2009; 16:131-6; PMID:19242650; http://dx.doi.org/ 10.1007/s00534-009-0064-z [DOI] [PubMed] [Google Scholar]
- 29. Lakey JR, Burridge PW, Shapiro AM. Technical aspects of islet preparation and transplantation. Transpl Int 2003; 16:613-32; PMID:12928769; http://dx.doi.org/ 10.1111/j.1432-2277.2003.tb00361.x [DOI] [PubMed] [Google Scholar]
- 30. Johnson PR, White SA, London NJ. Collagenase and human islet isolation. Cell Transplant 1996; 5:437-52; PMID:8800512; http://dx.doi.org/ 10.1016/0963-6897(95)02050-0 [DOI] [PubMed] [Google Scholar]
- 31. Kin T, Zhai X, Murdoch TB, Salam A, Shapiro AM, Lakey JR. Enhancing the success of human islet isolation through optimization and characterization of pancreas dissociation enzyme. Am J Transplant 2007; 7:1233-41; PMID:17359501; http://dx.doi.org/ 10.1111/j.1600-6143.2007.01760.x [DOI] [PubMed] [Google Scholar]
- 32. McCarthy RC, Breite AG, Green ML, Dwulet FE. Tissue dissociation enzymes for isolating human islets for transplantation: factors to consider in setting enzyme acceptance criteria. Transplantation 2011; 91:137-45; PMID:21116222; http://dx.doi.org/ 10.1097/TP.0b013e3181ffff7d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ricordi C, Lakey JR, Hering BJ. Challenges toward standardization of islet isolation technology. Transplant Proc 2001; 33:1709; PMID:11267479; http://dx.doi.org/ 10.1016/S0041-1345(00)02651-8 [DOI] [PubMed] [Google Scholar]
- 34. Hanley SC, Paraskevas S, Rosenberg L. Donor and isolation variables predicting human islet isolation success. Transplantation 2008; 85:950-5; PMID:18408573; http://dx.doi.org/ 10.1097/TP.0b013e3181683df5 [DOI] [PubMed] [Google Scholar]
- 35. Lakey JR, Warnock GL, Shapiro AM, Korbutt GS, Ao Z, Kneteman NM, Rajotte RV. Intraductal collagenase delivery into the human pancreas using syringe loading or controlled perfusion. Cell Transplant 1999; 8:285-92; PMID:10442741 [DOI] [PubMed] [Google Scholar]
- 36. Bucher P, Mathe Z, Bosco D, Andres A, Kurfuerst M, Ramsch-Gunther N, Buhler L, Morel P, Berney T. Serva collagenase NB1: a new enzyme preparation for human islet isolation. Transplant Proc 2004; 36:1143-4; PMID:15194398; http://dx.doi.org/ 10.1016/j.transproceed.2004.04.023 [DOI] [PubMed] [Google Scholar]
- 37. Nano R, Clissi B, Melzi R, Calori G, Maffi P, Antonioli B, Marzorati S, Aldrighetti L, Freschi M, Grochowiecki T, et al. . Islet isolation for allotransplantation: variables associated with successful islet yield and graft function. Diabetologia 2005; 48:906-12; PMID:15830183; http://dx.doi.org/ 10.1007/s00125-005-1725-3 [DOI] [PubMed] [Google Scholar]
- 38. Balamurugan AN, Loganathan G, Bellin MD, Wilhelm JJ, Harmon J, Anazawa T, Soltani SM, Radosevich DM, Yuasa T, Tiwari M, et al. . A new enzyme mixture to increase the yield and transplant rate of autologous and allogeneic human islet products. Transplantation 2012; 93:693-702; PMID:22318245; http://dx.doi.org/ 10.1097/TP.0b013e318247281b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ichii H, Pileggi A, Molano RD, Baidal DA, Khan A, Kuroda Y, Inverardi L, Goss JA, Alejandro R, Ricordi C. Rescue purification maximizes the use of human islet preparations for transplantation. Am J Transplant 2005; 5:21-30; PMID:15636608; http://dx.doi.org/ 10.1111/j.1600-6143.2005.00698.x [DOI] [PubMed] [Google Scholar]
- 40. Ichii H, Wang X, Messinger S, Alvarez A, Fraker C, Khan A, Kuroda Y, Inverardi L, Goss JA, Alejandro R, et al. . Improved human islet isolation using nicotinamide. Am J Transplant 2006; 6:2060-8; PMID:16827790; http://dx.doi.org/ 10.1111/j.1600-6143.2006.01452.x [DOI] [PubMed] [Google Scholar]
- 41. Ponte GM, Pileggi A, Messinger S, Alejandro A, Ichii H, Baidal DA, Khan A, Ricordi C, Goss JA, Alejandro R. Toward maximizing the success rates of human islet isolation: influence of donor and isolation factors. Cell Transplant 2007; 16:595-607; PMID:17912951; http://dx.doi.org/ 10.3727/000000007783465082 [DOI] [PubMed] [Google Scholar]
- 42. Qin H, Matsumoto S, Klintmalm GB, De Vol EB. A meta-analysis for comparison of the two-layer and university of Wisconsin pancreas preservation methods in islet transplantation. Cell Transplant 2011; 20:1127-37; PMID:21092403; http://dx.doi.org/ 10.3727/096368910X544942 [DOI] [PubMed] [Google Scholar]
- 43. Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005; 331:897-900; PMID:16223826; http://dx.doi.org/ 10.1136/bmj.331.7521.897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151:264-9, W64; PMID:19622511; http://dx.doi.org/ 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 45. Coleman CI, Phung OJ, Cappelleri JC, Baker WL, Kluger J, White CM, Sobieraj DM. Use of Mixed Treatment Comparisons in Systematic Reviews. Rockville (MD), 2012. [PubMed] [Google Scholar]
- 46. Spiegelhalter DJ, Best NG, Carlin BP, Van Der Linde A. Bayesian measures of model complexity and fit. J Royal Stat Society 2002; 64:583-639; http://dx.doi.org/ 10.1111/1467-9868.00353 [DOI] [Google Scholar]
- 47. Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011; 64:163-71; PMID:20688472; http://dx.doi.org/ 10.1016/j.jclinepi.2010.03.016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


