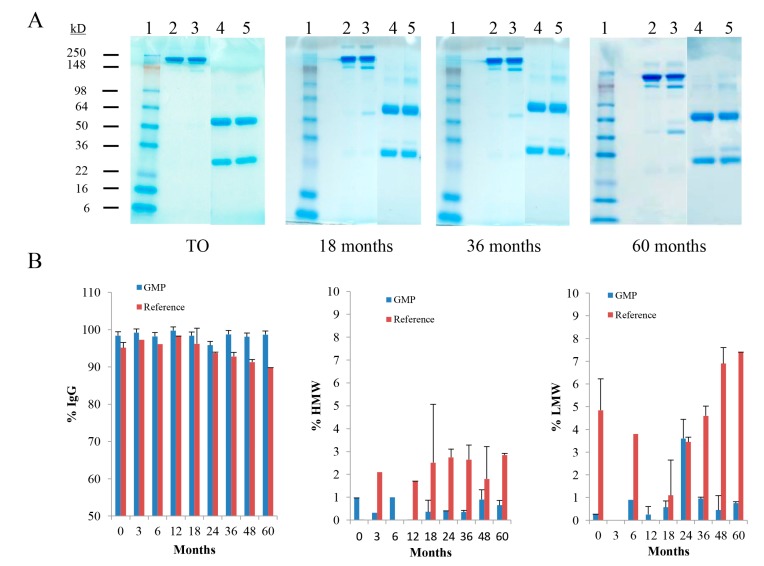
Figure 2.
Analysis of cGMP TCMC-trastuzumab integrity and purity. Over a 60 month period the cGMP TCMC-trastuzumab was analyzed at T0, 3, 6, 12, 18, 24, 36, 48 and 60 months using SDS-PAGE and by SE-HPLC. Panel A: Representative images spanning the study period are shown with cGMP in Lanes 2 (non-reduced) and Lane 4 (reduced); the Reference TCMC-trastuzumab in Lanes 3 (non-reduced) and Lane 5 (reduced). Lane 1 contains the molecular weight standard. Panel B: The individual graphs from the SE-HPLC data depict the percentage of the IgG at ~15 min, the percentage of HMW species at ~12.6 min for monitoring of aggregate formation and the percentage of LMW species at ~21.4 min as an indicator of degradation. Values are the average of two experiments and the error bars represent the standard deviation.