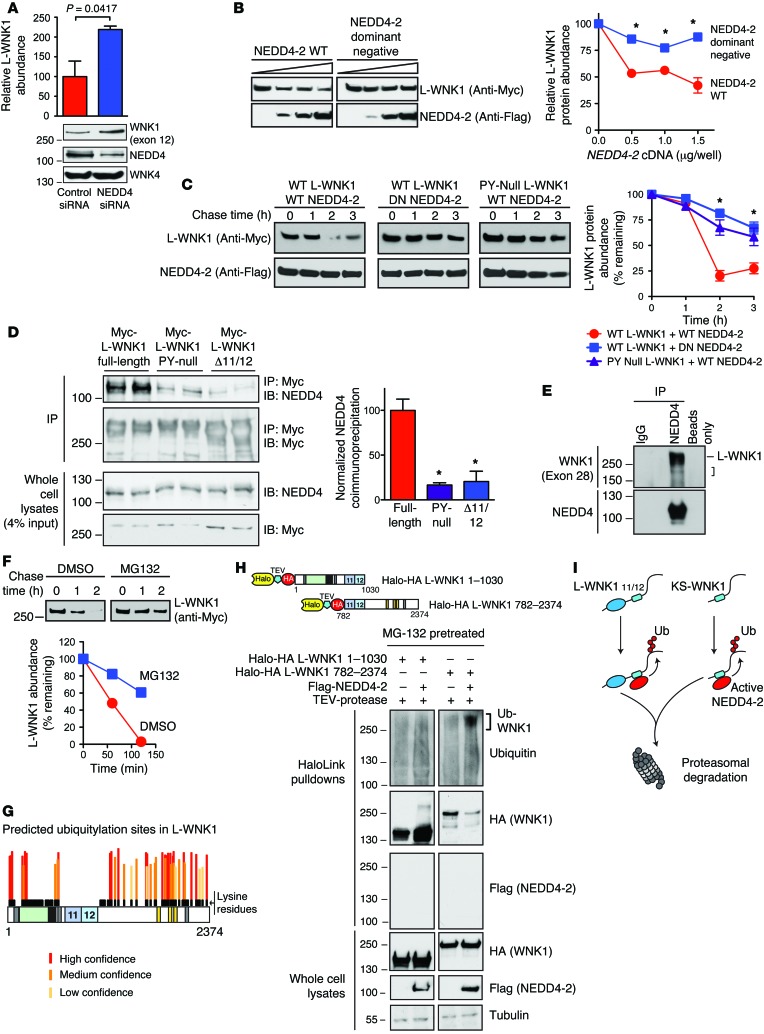
Figure 5. PY motif–containing WNK1 isoforms are NEDD4-2 substrates.
(A) Pan-NEDD4 knockdown increases WNK1 abundance in HEK-293T cells. n = 5; P = 0.0417 by Student’s t test. (B) WT NEDD4-2 (0–1.5 μg/well) decreased L-WNK1 abundance (0.5 μg/well) in HEK-239T cells; dominant-negative NEDD4-2 (DN; C938S) had no effect. n = 4; *P < 0.01 by Student’s t test for the indicated NEDD4-2 dosages. (C) CHX chases in HEK-293T cells. WT NEDD4-2 destabilized WT Myc-L-WNK1 to a greater degree than DN Nedd4-2. PY motif–null L-WNK1 was resistant to WT NEDD4-2. n = 4; *P < 0.01, by 1-way ANOVA, Dunnett’s test. (D) Coimmunoprecipitation assay. Left: Anti-Myc immunoprecipitates (IP) from HEK-293T cells transiently expressing Myc-tagged L-WNK1 constructs were immunoblotted (IB) for endogenous NEDD4 isoforms. Right: Quantification of IP results. n = 4; *P < 0.001, by 1-way ANOVA, Dunnett’s test. (E) Endogenous co-IP of WNK1 with NEDD4 isoforms in mpkCCDc14 cells. L-WNK1 and lower MW species (bracket) were detected. Representative of 3 experiments. (F) MG-132 (10 μM) attenuates NEDD4-2 mediated L-WNK1 degradation by CHX chase in HEK-293T cells. Representative of 3 experiments. (G) Putative ubiquitylation sites on L-WNK1, stratified by UbPred. Lysine residues are shown in black. Notably, exons 11 and 12 are completely lysine deficient. (H) In vivo ubiquitylation assay. Top: N- and C-terminal Halo-HA double-tagged L-WNK1 cDNAs with TEV cleavage spacer. Bottom: Lysates of MG-132–pretreated HEK293T cells expressing WNK1 fragments +/– Flag–NEDD4-2 were incubated with HaloLink resin. Following covalent pulldown, fragments were washed with SDS to remove autoubiquitylated NEDD4-2 (67), cleaved with TEV, and subjected to immunoblotting. Representative of 4 experiments. (I) NEDD4-2 ubiquitylates the WNK1 C-terminus common to both L- and KS-WNK1, marking both species for degradation. See also Supplemental Figures 4 and 5.