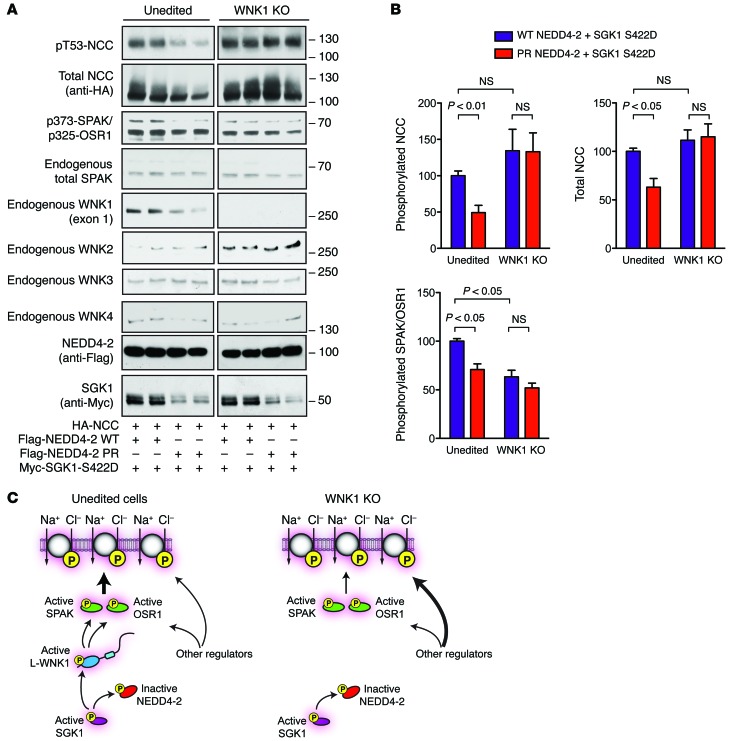
Figure 7. WNK1 is necessary for NEDD4-2 and SGK1 to modulate NCC abundance and phosphorylation in HEK-293 cells.
(A) WNK1 KO HEK-293T cells and unedited controls were transfected with NCC (1 μg), SGK1-S422D (1 μg), and either WT or phosphorylation-resistant (PR) NEDD4-2 (1 μg). Thirty-six hours after transfection, the cells were lysed and subjected to immunoblotting with the indicated antibodies. (B) Quantification of total NCC, phosphorylated NCC, and phosphorylated SPAK/OSR1 signal in A. n = 4; P values as indicated by 1-Way ANOVA Bonferroni multiple-comparisons post hoc test. (C) Proposed model of the effects of WNK1 deletion on NEDD4-2/SGK1 regulation of NCC in HEK-293T cells. In unedited cells, WT NEDD4-2 interacts with L-WNK1. This interaction can be disrupted by SGK1 phosphorylation at previously defined major and minor sites, increasing L-WNK1 abundance, SPAK/OSR1 phosphorylation, and NCC activation. In WNK1 KO cells, NEDD4-2 and SGK1 cannot alter NCC phosphorylation status. Other regulators, possibly WNK complexes that are less NEDD4-2 sensitive, maintain NCC activity through compensation.