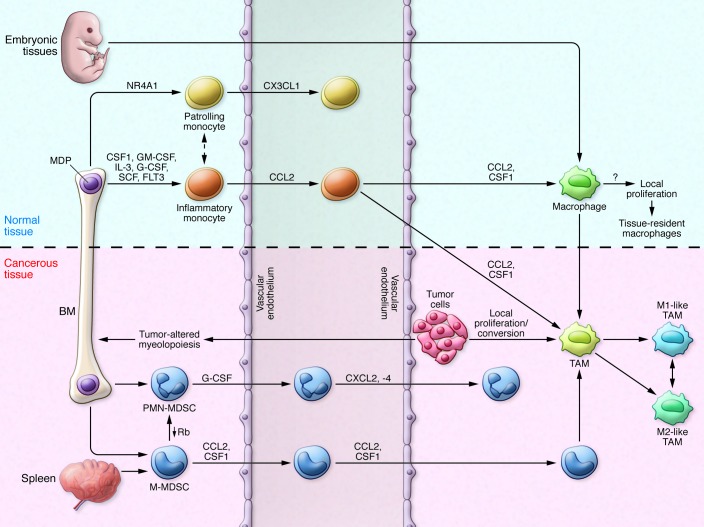
Figure 2. MDSC and TAM development in tumor-bearing mice.
Under steady-state conditions, resident macrophages may originate from either embryonic tissues or inflammatory monocytes. Resident macrophages are programmed by local factors, and molecular switches support their differentiation. Circulating monocytes can be divided into two subsets: patrolling monocytes (Ly6CloCX3CR1hi) and inflammatory monocytes (Ly6ChiCD11b+CD11c–MHCII–VCAM1–CCR2+), originating from macrophage and DC precursors (MDPs) in BM. Inflammatory monocytes migrate from blood to tissue under the guidance of CCL2/CCR2 chemokine signaling. Tumor cells secrete several factors that modify physiological myelopoiesis, promoting MDP differentiation into PMN-MDSCs (CD11b+Ly6G+) and MO-MDSCs (CD11b+Ly6ChiCCR2+CD115+F4/80lo). MO-MDSCs also originate from the spleen under conditions of emergency and reactive myelopoiesis. MO-MDSCs and inflammatory monocytes migrate to tumor tissues via CCL2/CCR2 and CSF1 signaling and differentiate into TAMs (Ly6C–CD11b+/loCD68+CD1d+MHCIIhi/loF4/80+VCAM1+) in the presence of specific signals released by tumor cells within the local environment. However, the TAM phenotypic profile depends on cancer histology and stage, which might influence marker distribution. TAMs also proliferate locally, with different rates in various tumors. Furthermore, TAMs are inherently plastic, with an activation state falling along a continuum between the two extremes of M1- and M2-like phenotypes. Rb, retinoblastoma.