Abstract
Steroids are used in the management of drug-induced acute interstitial nephritis (AIN). The present study was undertaken to compare the efficacy of pulse methyl prednisolone with oral prednisolone in the treatment of drug-induced AIN. Patients with biopsy-proven AIN with a history of drug intake were randomized to oral prednisolone (Group 1) 1 mg/kg for 3 weeks or a pulse methyl prednisolone (Group II) 30 mg/kg for 3 days followed by oral prednisolone 1 mg/kg for 2 weeks, tapered over 3 weeks. Kidney biopsy scoring was done for interstitial edema, infiltration and tubular damage. The response was reported as complete remission (CR) (improvement in estimated glomerular filtration rate [eGFR] to ≥60 ml/min/1.73 m2), partial remission (PR) (improvement but eGFR <60 ml/min/1.73 m2) or resistance (no CR/PR). A total of 29 patients, Group I: 16 and Group II: 13 were studied. Offending drugs included nonsteroidal anti-inflammatory drugs, herbal drugs, antibiotics, diuretic, rifampicin and omeprazole. There was no difference in the baseline parameters between the two groups. The biopsy score in Groups I and II was 5.9 ± 1.1 and 5.1 ± 1.2, respectively. At 3 months in Group I, eight patients each (50%) achieved CR and PR. In Group II, 8 (61%) achieved CR and 5 (39%) PR. This was not significantly different. Percentage fall in serum creatinine at 1 week (56%) was higher in CR as compared to (42%) those with PR. (P = 0.14). Patients with neutrophil infiltration had higher CR compared to patients with no neutrophil infiltration (P = 0.01). Early steroid therapy, both oral and pulse steroid, is equally effective in achieving remission in drug-induced AIN.
Keywords: Acute interstitial nephritis, estimated glomerular filtration rate, nonsteroidal anti-inflammatory drugs
Introduction
Acute interstitial nephritis (AIN) is characterized by the presence of edema and inflammatory infiltrates within the interstitium and accounts for 15–27% of lesions in patients with acute kidney injury (AKI).[1] AIN is underreported as not all patients with suspected AIN undergo kidney biopsy and further AKI may be attributed to other causes.
Drug-induced AIN accounts for more than three-fourth of the cases of AIN. Majority of the drug-induced AIN are caused by either nonsteroidal anti-inflammatory drugs (NSAIDs) or antibiotics (mainly beta-lactam group of drugs). Drug-induced AIN is characterized by fever, rash and eosinophilia, but this triad is seen in minority of patients. Fever, rash and eosinophilia were seen in 27–36%, 15–22% and 23–35% of patients, respectively with AIN and about one-third may need dialytic support.[1,2,3] Eosinophiluria is associated with AIN but it does not distinguish AIN from other causes of AKI.[4] AIN is characterized by cellular infiltrates mainly comprised of T-lymphocytes and macrophages; with time the inflammatory infiltrate transforms to destructive fibrosis; fibrogenesis is demonstrable usually after 1 week of the disease,[5] so the treatment should be instituted as soon as the diagnosis of AKI due to AIN is made to prevent chronic renal impairment. The treatment includes stopping the offending drug along with steroid therapy. Steroids are initiated at high doses followed by a rapid taper off. Both pulse methylprednisolone followed by oral prednisolone (0.5–1 mg/kg) and oral prednisolone (1 mg/kg) only followed by rapid taper have been successfully tried in AIN.[1,6] There have been no formal comparisons or any prospective study of different steroid therapy used in AIN. So the present study was undertaken to compare the efficacy of pulse methyl prednisolone therapy with oral prednisolone therapy in the treatment of AKI due to drug-induced AIN.
Materials and Methods
The randomized control study was conducted at Department of Nephrology, PGIMER, Chandigarh over a 2-year period. All patients with biopsy-proven AIN with a history of drug ingestion were included in the study. Patients with lupus nephritis, multiple myeloma, infection, chronic hepatitis B and C, patients who received some sort of immunosuppressant before undergoing the biopsy, patients with history of offending drug consumption > 6 months, and patients with tubular atrophy and interstitial fibrosis occupying > 50% of the biopsy area were excluded from the study.
A detailed clinical history was elicited with special reference to duration of drug exposure, duration of symptoms, rash, presence of oliguria (defined below), hematuria and anasarca were recorded. Urine examination was carried out, which included urine routine examination and urine for eosinophils (Giemsa stain). Complete hemogram, renal function tests (blood urea, serum creatinine, serum sodium and potassium), liver function tests (aspartate aminotransferase, aminotransferase, total bilirubin, direct bilirubin and alkaline phosphatase), antinuclear antibody, anti-neutrophil cytoplasmic antibody, hepatitis B surface antigen, anti-hepatitis C antibody and human immunodeficiency virus-I/II were carried out at the time of admission. Estimated glomerular filtration rate (eGFR) was calculated using modification of diet in renal disease formulae. Kidney biopsy was performed at the clinical suspicion of drug-induced AIN. Light microscopy and immunofluorescence was carried on all kidney biopsy tissue. Single pathologist graded all the renal histology as per scoring system mentioned in Table 1. Interstitial edema was graded based on the area of the cortex occupied by the edema as mild (10–30%), moderate (31–60%) and severe (61–100%). Nature of cellular infiltrate was further analysed semi-quantitatively by the presence of various cells like lymphocytes, eosinophils, neutrophils and plasma cells. The results were expressed as mild (10–30%), moderate (31–60%) or severe (61–100%) interstitial inflammation. Tubular damage was assessed, as absent when tubules were normal, mild when tubular degeneration and regeneration were present in 10–30% of the biopsy area and moderate and severe when tubular degeneration and regeneration were seen in 31–60% and >60% of the biopsy area, respectively.
Table 1.
Histological score

Treatment groups
When renal functions did not improve within 1 week of stopping the offending drug, patients were randomized using computer-generated random numbers into two groups.
Group 1: Received oral prednisolone 1 mg/kg for 3 weeks followed by rapid tapering in the next 3 weeks.
Group II: Received intravenous methyl prednisolone 30 mg/kg (maximum 1 g) as slow intravenous infusion over 60 min for 3 consecutive days followed by oral prednisolone 1 mg/kg for 2 weeks and tapered in next 3 weeks.
Additional supportive treatment including dialysis was given as required.
Response
Patients were followed up to 12 weeks after completing the therapy. Patients were monitored with renal function weekly for the first 3 weeks and then every 3 weeks till 3 months. Response was classified as complete, partial or nonresponder (defined below).
Definitions
Complete remission (CR): Improvement in eGFR to ≥60 ml/min/1.73 m2 at the end of 3 months of follow-up. Partial remission: Improvement of eGFR, but <60 ml/min/1.73 m2 at the end of follow-up. Nonresponders: No improvement in eGFR from baseline. Oliguria: 24-h urine output <400 ml. Eosinophiluria: >1% eosinophils in the urine among all leukocytes.
Statistical analysis
All results were expressed as mean ± standard deviation for continuous variables. Responders (complete and partial) in both the groups were compared using Chi-square test. Student's t-test was used to compare the serum creatinine and eGFR. P <0.05 was considered significant.
Results
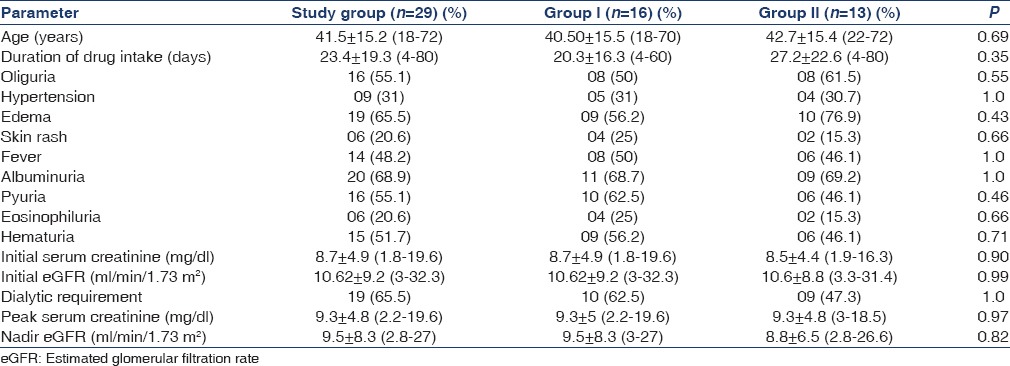
The study included 29 patients (11 female and 18 male). The mean age was 41.5 ± 15.2 years (range 18–72). The offending drug included rifampicin in 2 (6.9%) patients, NSAIDs in 9 (31%), NSAIDs in combination with antibiotic and rifampicin 1 (3.4%) each, herbal medicines in 8 (27.5%), furosemide 2 (6.9%), antibiotics in 5 (17.2%) and omeprazole in 1 (3.4%) case. The duration of exposure before presenting with renal dysfunction was 23.4 ± 19.3 (4–80) days. Oliguria was seen in 16 (55.1%), hypertension in 09 (31%), edema in 19 (65.5%), skin rashes in 6 (20.6%) and fever in 14 (48.2%). There was need of renal replacement therapy at presentation in 19 (65.5%) patients. Urine examination revealed albuminuria in 20 (68.9%), hematuria in 15 (51.7%), pyuria in 16 (55.1%) and eosinophiluria in 6 (20.6%) patients. Baseline serum creatinine at the diagnosis of renal dysfunction was 8.6 ± 4.6 (1.8–19.6) mg/dl. Peak serum creatinine was 9.3 ± 4.8 (2.2–19.6) mg/dl. The baseline eGFR and nadir eGFR were 10.6 ± 8.9 (3.3–32.3) ml/min/1.73 m2 and 9.2 ± 7.4 (3–27) ml/min/1.73 m2, respectively. There were no significant differences in the above-mentioned parameters in methyl prednisolone and oral prednisolone group [Table 2].
Table 2.
Baseline parameters of the study population
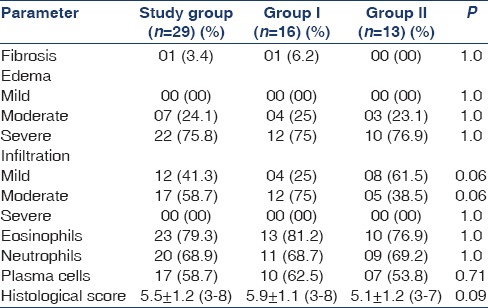
Presence of tubular atrophy and interstitial fibrosis was seen in 1 (3.4%) patient. Moderate and severe interstitial edema was seen in 7 (24.1%) and 22 (75.8%) patients, respectively. Interstitial inflammation of mild and moderate severity was seen in 12 (41.3%) and 17 (58.7%) patients, respectively. Presence of eosinophils, neutrophils and plasma cells in the interstitial infiltrate was seen in 23 (79.3), 20 (68.9%) and 17 (58.7%) patients, respectively. Histological score was 5.5 ± 1.2 (3–8) [Table 3]. Details of the histopathological parameters of both groups (I and II) are mentioned in Table 3; both the groups were comparable with no significant differences in the baseline biopsy.
Table 3.
Histopathology of the study groups
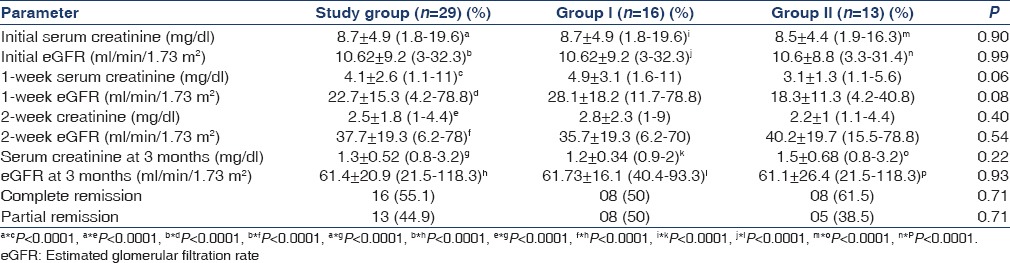
A total of 29 patients fulfilled the inclusion criteria and completed the study. Sixteen (55.1%) and 13 (44.9%) patients were randomized to receive oral (Group I) and pulse steroids (Group II), respectively. After initiation of the treatment, the serum creatinine significantly reduced to 4.1 ± 2.6 (1.1–11) and 2.5 ± 1.8 (1–4.4) mg/dl (P < 0.0001) at the end of 1 week and 2 week of therapy, respectively. The eGFR also improved significantly compared to baseline to 22.7 ± 15.3 (4.2–78.8) (P < 0.0001) and 37.7 ± 19.3 (6.2–78) ml/min/1.73 m2 (P < 0.0001) at the end of 1 week and 2 weeks of therapy, respectively. At the completion of the study, the serum creatinine reduced further to 1.3 ± 0.52 (0.8–3.2) mg/dl with no patients requiring renal replacement therapy (P < 0.0001). The eGFR also settled at 61.4 ± 20.9 (21.5–118.3) ml/min/1.73 m2 at the completion of study (P < 0.0001). There was significant reduction in serum creatinine and increase in eGFR at 3 months of therapy compared to 2 weeks of therapy (P < 0.0001). Sixteen (55.1%) patients had CR and 13 (44.9%) had partial remission (PR) [Table 4]. There were no nonresponders in both the groups. The 1-week serum creatinine and eGFR in Group I and Group II was 4.9 ± 3.1 mg/dl and 3.1 ± 1.3 mg/dl (P = 0.06) and 28.1 ± 18.2 and 18.3 ± 11.3 ml/min/1.73 m2 (P = 0.08), respectively and there was a trend for lower serum creatinine and high eGFR in the pulse group, but was not statistically significant. The 2-week serum creatinine in Group I and Group II was 2.8 ± 2.3 mg/dl and 2.2 ± 1 mg/dl, respectively and there wasn’t any significant difference (P = 0.40). The eGFR in Group I and II at the end of 2 weeks of therapy was 35.7 ± 19.3 and 40.2 ± 19.7 ml/min/1.73 m2, respectively and there was no statistically significant difference (P = 0.54). There was a significant improvement in serum creatinine from baseline in both groups to 1.2 ± 0.34 mg/dl and 1.5 ± 0.6 mg/dl, respectively (P < 0.0001) at the end of 3 months. There was a significant increase in eGFR at the end of therapy compared to baseline and at 2 weeks in both Group I and Group II to 61.73 ± 16.1 and 61.1 ± 26.4 ml/min/1.73 m2 (P < 0.0001), respectively [Table 4].
Table 4.
Outcome of patients with steroid therapy
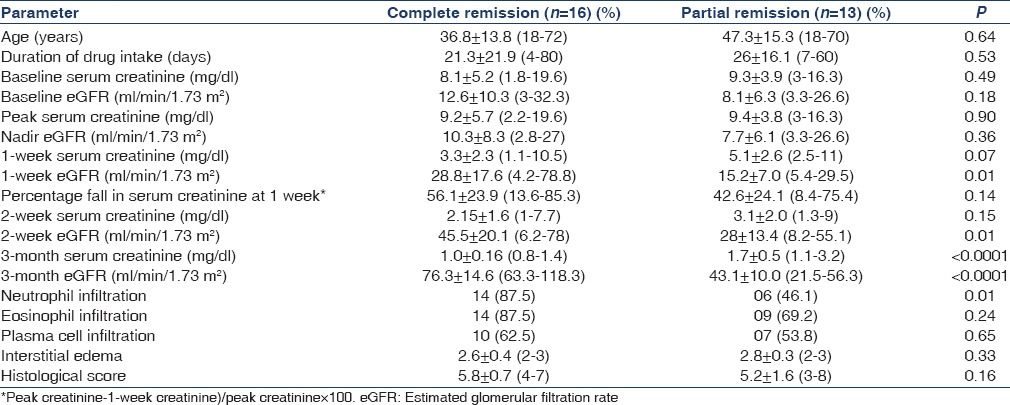
On comparing various parameters in patients with CR and PR, it was observed that eGFR was significantly higher after 1 week of therapy in those with CR as compared to PR. The mean percentage fall in serum creatinine at 1 week compared to the peak serum creatinine was 56.1 ± 23.9 (13.6–85.3)% in those with CR as compared to 42.6 ± 24.1 (8.4–75.4)% in those with PR (P = 0.14). Patients with CR had higher neutrophil infiltration in the interstitium compared to patients with PR (P = 0.01) [Table 5].
Table 5.
Comparison of the parameters among patients with complete remission and partial remission
A total of 8 patients (27.5%) experienced side effects with steroid therapy. Four (13.7%) patients (2 [12.5%] in Group I and 2 [15.3%] in Group II P > 0.05) developed infectious complication. Three (10.3%) patients (1 [6.25%] in Group I and 2 [15.3%] in Group II P > 0.05) had gastritis-requiring proton pump inhibitors and one patient (7.6%) in Group II developed diabetes mellitus, whose blood glucose normalized after stopping steroids at 3 weeks. There was no difference in the complication between both the groups.
Discussion
The role of steroids in the management of drug-induced AIN is not clear. However, many retrospective studies have shown significant benefit in term of reduction in serum creatinine and diuresis with steroids. There are no prospective or randomized trials available till date to validate the above statement.
There are at least three large retrospective studies of steroids in AIN. The first study was by Clarkson et al.[7] in 2004, the second study was by González et al. in 2008 and the third was by Muriithi et al.[6,8] Clarkson et al. had compared corticosteroids with conservative management in drug-induced AIN. The authors had analyzed results at 1, 6 and 12 months of therapy. Of the 42 patients with complete data available, 60% received corticosteroids and 40% received conservative treatment. At the end of follow-up there were no significant differences in the serum creatinine in patients managed with corticosteroids and conservative management. However, the median duration for starting corticosteroids was 3 weeks in the study. González et al. had carried out a retrospective analysis of 61 patients with drug-induced AIN. Eight-five percent of the patients received steroids and 15% were on conservative management. The need of chronic dialysis was significantly lower in patients treated with steroids as compared to those managed with conservative strategy (3.8 vs. 44%; P < 0.05). On further analysis, 53% of the patients achieved their baseline renal functions and 47% had persisting renal dysfunction. Patients with incomplete recovery of renal function had a significantly longer interval between withdrawal of the offending drug and starting steroids treatment (34 ± 17 vs. 13 ± 10 days; P < 0.05). On multivariate analysis, >7 days of starting steroids was associated with 6 times the risk of chronic renal dysfunction. In the present study, all the patients were started on corticosteroid therapy by 1 week of stopping the offending drug, 16 (55%) patients had complete recovery and 13 (45%) had partial recovery with some persisting chronic renal dysfunction. The results of the present study are similar to the study by González et al.[6] Muriithi et al.[8] had carried out a retrospective analysis of 133 patients with AIN, of which 70% of the etiology were drug-induced. At the end of 6 months, 88% of the drug-induced AIN patients treated with steroids had remission. Forty-nine percent had CR and 39% had PR. On subgroup analysis, patients with delay in starting steroid therapy (11 days) faired poorer than cases in whom steroid therapy was started earlier (8 days). The results of the present study are similar to that reported by Muriithi et al. All the patients in the present study were started on steroids if there was no recovery of renal functions after 1 week of cessation of the drug. The 100% recovery in the present study might be due to early start of the steroid therapy like that reported in the subgroup analysis of the study by Muriithi et al.[8]
Both oral corticosteroids and pulse methylprednisolone have been successfully used in AIN;[1] however, there is no formal comparison of both the therapy available. In the present there were no significant differences between the two-regimen used at the end of the study. However, there was a trend of lower serum creatinine and improvement in eGFR at 1 week of pulse methyl prednisolone therapy compared to the oral prednisolone group. However, the benefit of pulse methyl prednisolone was short-lived with there being no difference in the 2nd week eGFR and serum creatinine in both the groups. This could be explained by the higher initial dose of the steroids delivered in the pulse methyl prednisolone arm.
On comparing patients with CR and PR, the differences in eGFR were apparent right from the end of 1st week of the therapy. There were no differences in the baseline parameters between the patients in both the groups. However, patients with CR had significant increase in eGFR and reduction in serum creatinine at 1 week compared to those with PR. Patients with CR had >50% fall in serum creatinine at 1 week (mean 56%) compared to patients with PR (mean 42%); the difference was however not statistically significant. As the study included only 29 patients, the relatively small number of patients enrolled in the study may explain the lack of significant difference between the patients with CR and PR. Rapidity of fall in serum creatinine and improvement in eGFR could predict response to steroids in patients with AIN. Patients with neutrophil infiltration in the interstitium seem to have good response to steroids than patients without neutrophil infiltration. This could be explained by probably the acute nature of the kidney injury in patients with neutrophil infiltration. The study is limited by relative small sample size and short duration of follow-up.
To conclude, the present study is the first randomized controlled trials of early steroid therapy in patients with drug-induced AIN and shows convincing evidence of successful early steroid therapy, both oral and pulse steroid therapy in the management of drug-induced AIN.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Praga M, González E. Acute interstitial nephritis. Kidney Int. 2010;77:956–61. doi: 10.1038/ki.2010.89. [DOI] [PubMed] [Google Scholar]
- 2.Baker RJ, Pusey CD. The changing profile of acute tubulointerstitial nephritis. Nephrol Dial Transplant. 2004;19:8–11. doi: 10.1093/ndt/gfg464. [DOI] [PubMed] [Google Scholar]
- 3.Kodner CM, Kudrimoti A. Diagnosis and management of acute interstitial nephritis. Am Fam Physician. 2003;67:2527–34. [PubMed] [Google Scholar]
- 4.Muriithi AK, Nasr SH, Leung N. Utility of urine eosinophils in the diagnosis of acute interstitial nephritis. Clin J Am Soc Nephrol. 2013;8:1857–62. doi: 10.2215/CJN.01330213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwarz A, Krause PH, Kunzendorf U, Keller F, Distler A. The outcome of acute interstitial nephritis: Risk factors for the transition from acute to chronic interstitial nephritis. Clin Nephrol. 2000;54:179–90. [PubMed] [Google Scholar]
- 6.González E, Gutiérrez E, Galeano C, Chevia C, de Sequera P, Bernis C, et al. Early steroid treatment improves the recovery of renal function in patients with drug-induced acute interstitial nephritis. Kidney Int. 2008;73:940–6. doi: 10.1038/sj.ki.5002776. [DOI] [PubMed] [Google Scholar]
- 7.Clarkson MR, Giblin L, O’Connell FP, O’Kelly P, Walshe JJ, Conlon P, et al. Acute interstitial nephritis: Clinical features and response to corticosteroid therapy. Nephrol Dial Transplant. 2004;19:2778–83. doi: 10.1093/ndt/gfh485. [DOI] [PubMed] [Google Scholar]
- 8.Muriithi AK, Leung N, Valeri AM, Cornell LD, Sethi S, Fidler ME, et al. Biopsy-proven acute interstitial nephritis, 1993-2011: A case series. Am J Kidney Dis. 2014;64:558–66. doi: 10.1053/j.ajkd.2014.04.027. [DOI] [PubMed] [Google Scholar]


