Figure 1.
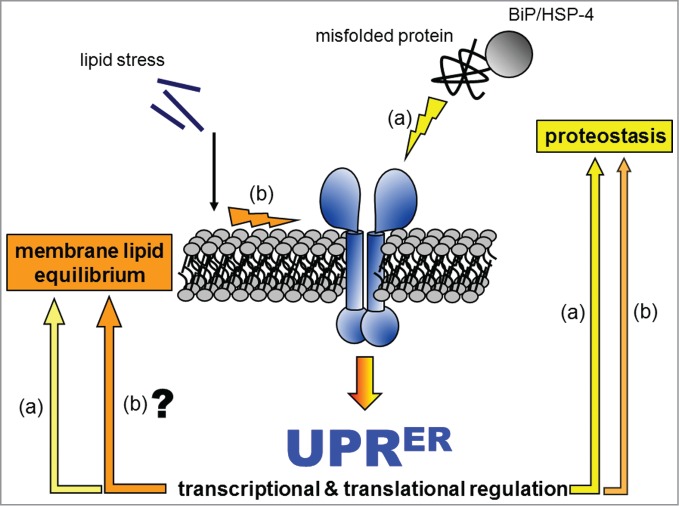
The UPRER engages different downstream outputs in response to distinct upstream inputs. Our hypothetical model of the UPRER incorporates independent sensing of proteostatic or lipid stress, which leads to distinctive downstream outputs that aim to alleviate the specific input stress. In one scenario (A), the UPRER sensors directly and/or indirectly sense the accumulation of misfolded protein in the ER. The resulting downstream outputs promote protein quality control by inducing the expression of chaperones and of the protein degradation machinery, by optimizing the redox environment for protein folding, and by attenuating general translation. In the other scenario (B), lipid stress imposed by free saturated fatty acids, decreased fatty acid desaturation, or reduced PC biosynthesis activates the UPRER sensors through its impact on the ER membrane. This type of UPRER input can occur either with or without misfolded-proteins, suggesting that there may be downstream adaptive outputs dedicated specifically to membrane lipid homeostasis. Simultaneously, lipid disequilibrium also activates pathways promoting protein quality control, as indicated by the activation of conventional UPRER markers (e.g., the chaperone BiP).
