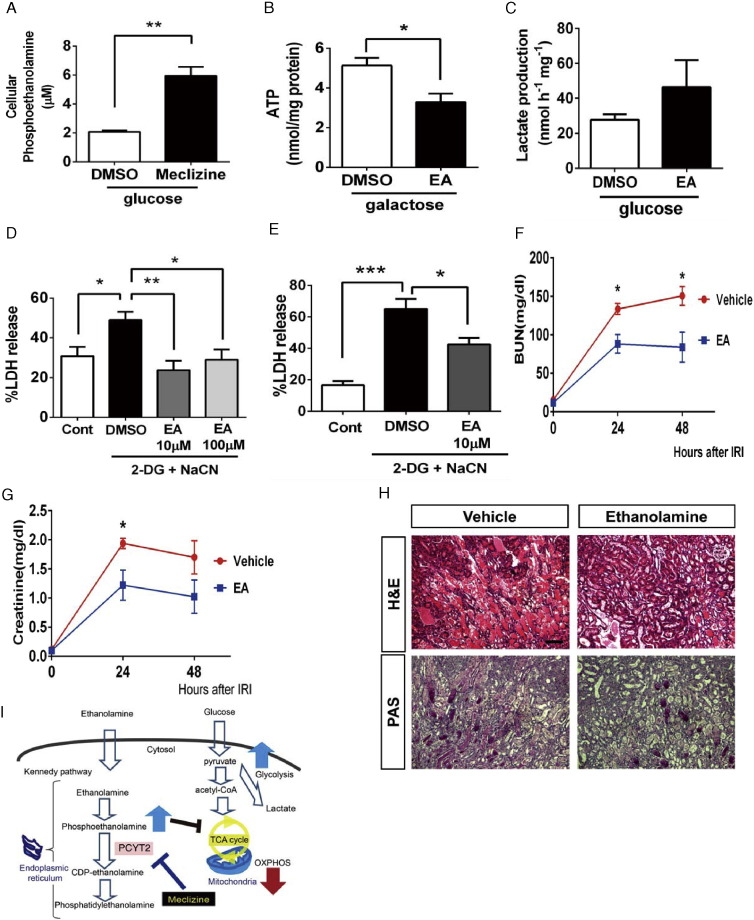
Fig. 7.
Cellular phosphoethanolamine is increased by meclizine and recapitulated meclizine-induced protection. (A) Phosphoethanolamine levels in HK-2 cells treated with or without 25 μM of meclizine for 17 h (n = 3). (B) Cellular ATP levels in HK-2 cells cultured in 10% DMEM containing 10 mM galactose with or without 10 μM of ethanolamine (EA) for 17 h (n = 4). (C) Lactate production in HK-2 cells after treatment with or without 10 μM of EA for 17 h (n = 7). (D) LDH release from HK-2 cells treated with either 10 or 100 μM of EA for 17 h followed by 2 hr of chemical anoxia (n = 7). (E) LDH release from LLC-PK1 cells treated with 10 μM of EA for 17 h followed by 2 hr of chemical anoxia (n = 4). (F) BUN and (G) serum creatinine concentrations at 24 and 48 h after IRI treated 2 h before, just after clamp removal and skin closure 24 h after ischemia with vehicle (n = 5) or EA (n = 4). (H) Representative images of H&E and PAS-stained kidney sections 48 h after IRI. Original magnification 100 ×, scale bar = 100 μm. (I) Summary of the mechanisms proposed for meclizine-induced protective effects against ischemic injury. Meclizine inhibits phosphate cytidylyltransferase 2 (PCYT2) and causes an increase in cytosolic phosphoethanolamine, a central precursor in the Kennedy pathway. High levels of intracellular phosphoethanolamine inhibit mitochondrial respiration.
**p < 0.01 and *p < 0.05. Statistical significance was determined using t test (A, B, C, F, G) or one-way ANOVA followed by Tukey's post-hoc test (D, E). The columns and error bars are the mean ± SEM.