Abstract
Pain is a significant problem in diseases affecting the spinal cord, including demyelinating disease. To date, studies have examined the reliability of clinical measures for assessing and classifying the severity of spinal cord injury (SCI) and also to evaluate SCI-related pain. Most of this research has focused on adult populations and patients with traumatic injuries. Little research exists regarding pediatric spinal cord demyelinating disease. One reason for this is the lack of reliable and useful approaches to measuring spinal cord changes since currently used diagnostic imaging has limited specificity for quantitative measures of demyelination. No single imaging technique demonstrates sufficiently high sensitivity or specificity to myelin, and strong correlation with clinical measures. However, recent advances in diffusion tensor imaging (DTI) and magnetization transfer imaging (MTI) measures are considered promising in providing increasingly useful and specific information on spinal cord damage. Findings from these quantitative imaging modalities correlate with the extent of demyelination and remyelination. These techniques may be of potential use for defining the evolution of the disease state, how it may affect specific spinal cord pathways, and contribute to the management of pediatric demyelination syndromes. Since pain is a major presenting symptom in patients with transverse myelitis, the disease is an ideal model to evaluate imaging methods to define these regional changes within the spinal cord. In this review we summarize (1) pediatric demyelinating conditions affecting the spinal cord; (2) their distinguishing features; and (3) current diagnostic and classification methods with particular focus on pain pathways. We also focus on concepts that are essential in developing strategies for the detection, monitoring, treatment and repair of pediatric myelitis.
Keywords: Inflammation, Chronic pain, MRI, DTI, MTI
Graphical abstract
Highlights
-
•
Pain is a major presenting symptom in children with myelitis.
-
•
Currently used imaging has limited sensitivity to myelin content.
-
•
We provide a summary on pediatric demyelinating conditions.
-
•
We review pain involvement and pathways affected by demyelination.
-
•
We review imaging modalities for the diagnosis and monitoring of myelitis.
1. Introduction
Injury to the spinal cord presents a significant clinical and therapeutic problem (Table 1) that includes significant neuropathic pain syndromes. While most spinal cord injuries are in adults, both traumatic and non-traumatic injuries present in children, with the most common being neoplasms and vascular (Citterio et al., 2004) and demyelinating diseases including acute transverse myelitis (Nair et al., 2005; Galvin et al., 2013). Transverse myelitis is “a neurological disorder caused by inflammation across both sides of one level, or segment, of the spinal cord” (National Institute of Neurological Disorders and Stroke (NINDS), 2015). The term ‘myelitis’ refers to inflammation of the spinal cord while the term ‘transverse’ refers to the location of the inflammation across the width of the spinal cord.
Table 1.
Incidence rates and etiology in non-traumatic spinal cord injuries.
| Sample characteristics |
Etiology (n) |
Pain assessment |
||||||
|---|---|---|---|---|---|---|---|---|
| Reference | Size (n) | Mean age (years) | Male/female ratio | Inflammatory | Vascular | Neoplasm | Degenerative | |
| McKinley et al., 1999 | 86 | 61.2 | 1:1 | 10 | 7 | 22 | Not available | Not performed |
| Scivoletto et al., 2003 | 177 | 54.0 | 1.7:1 | 40 | 36 | 39 | 61 | Reported by 42% |
| Citterio et al., 2004 | 330 | 55.2 | 1.7:1 | 63 | 81 | 81 | 60 | Not performed |
| New et al., 2005 | 70 | 69.0 | 0.8:1 | 12 | 10 | 23 | 18 | Not performed |
| Nair et al., 2005 | 297 | 32.0 | 1.07:1 | 192 | 3 | 85 | Not available | Reported by 49.3% |
| Galvin et al., 2013 | 68 | 8.3 | 1.8:1 | 15 | Not available | 40 | Not available | Not performed |
Acute transverse myelitis may occur as an isolated inflammatory process or as part of a chronic demyelinating disorder such as multiple sclerosis (MS), neuromyelitis optica (NMO), acute disseminated encephalomyelitis (ADEM) or a syndrome characterized as polio-like myelitis or acute flaccid myelitis (Greninger et al., 2015; Pfeiffer et al., 2015; Mirand and Peigue-Lafeuille, 2015). Transverse myelitis can be classified into two types: complete or partial. Complete transverse myelitis usually involves major bilateral loss in motor, sensory and sphincter function. It can be associated with a long spinal cord lesion exceeding three vertebral bodies in length, referred to as Longitudinally Extensive Transverse Myelitis (LETM).
Damage resulting from the inflammation to the fibers in the spinal cord tracts results in a constellation of symptoms and signs characteristic of spinal cord damage including pain, weakness or paralysis, urinary retention and loss of control of bowel function. In some patients there is complete recovery, while in others, these symptoms, including pain, may persist for years. Routine Magnetic Resonance Imaging (MRI) has been the imaging modality of choice in the detection of neuroinflammation; however, studies have shown it to have poor correlation with clinical status of patients with demyelinating injuries (Verhey and Banwell, 2013; Alper et al., 2011; Pidcock et al., 2007; Banwell et al., 2009). Finding more sensitive approaches to defining the location, severity and evolution (i.e., persistence or recovery) of regional demyelination may contribute to a more informed approach to diagnosis and treatment.
Pain in spinal cord disease is frequently severe and disabling (Cardenas and Felix, 2009). The incidence of pain is reported in approximately 65% of patients with chronic SCI, with nearly one third of these patients rating their pain as severe (Yezierski, 1996; Siddall and Loeser, 2001; Schomberg et al., 2012). The pain may be musculoskeletal, neuropathic and less commonly visceral. In spinal cord demyelination, pain is believed to develop following damage to the spinothalamic pathways that under normal condition carry nociceptive information to the brain (Defresne et al., 2003; Siddall et al., 1997). Symptomatically, these changes result in pain at and below the level of the damage.
In this review we summarize (1) spinal cord demyelinating conditions in the pediatric population; (2) their distinguishing features; and (3) classification methods in spinal cord disease with particular focus on pain. We also focus on the current and developing approaches to objective measures of spinal cord damage and recovery using different MRI methods.
1.1. Search terms and methodology
English language literature search of demyelination of spinal cord in children and MRI measures in pain was undertaken using PubMed (http://www.ncbi.nlm.nih.gov/pubmed) from inception until March 2015. Keywords included the terms ‘demyelination’, ‘myelitis’, ‘spinal cord’, ‘pediatric’, ‘neuropathic pain’ and ‘imaging’. Of note, using search terms “pediatric AND myelitis AND pain AND diffusion” yielded no results suggesting a paucity of information in the field.
2. Pediatric spinal cord demyelination
Inflammatory demyelinating myelopathies represent the majority of pediatric non-traumatic SCI (Nair et al., 2005; Verhey and Banwell, 2013). These are often grouped under the term “myelitis” which comprises acute transverse myelitis, NMO and spinal cord relapses in MS. Each year, an estimated 1400 new cases of acute transverse myelitis occur in the United States (Banwell et al., 2009). Among these cases, 20% are reported in children less than 18 years of age (Alper et al., 2011). Transverse myelitis was believed to affect males and females equally with a reported 1.04 ratio (Pidcock et al., 2007). However a more recent study showed a slight male predominance (ratio = 1:0.9) in children less than 10 years of age and a female predominance (ratio = 1:1.2) in patients older than 10 years (Banwell et al., 2009). The reason for these disparities remains unclear. Demyelination is believed to be the pathological basis in transverse myelitis and presents acutely in children, with symptoms appearing over 24–48 h (National Institute of Neurological Disorders and Stroke (NINDS), 2015; Chitnis, 2013).
2.1. Diagnosis of transverse myelitis
In 2002, the Transverse Myelitis Consortium Working Group established criteria for diagnosis of acute transverse myelitis (Transverse Myelitis Consortium Working Group, 2002). The diagnosis requires patients to undergo lumbar puncture to assess Cerebrospinal Fluid (CSF) white blood cells and IgG index and un-enhanced or gadolinium-enhanced MRI of the brain and spinal cord (Kerr and Ayetey, 2002; Jacob and Weinshenker, 2008; Pittock and Lucchinetti, 2006).
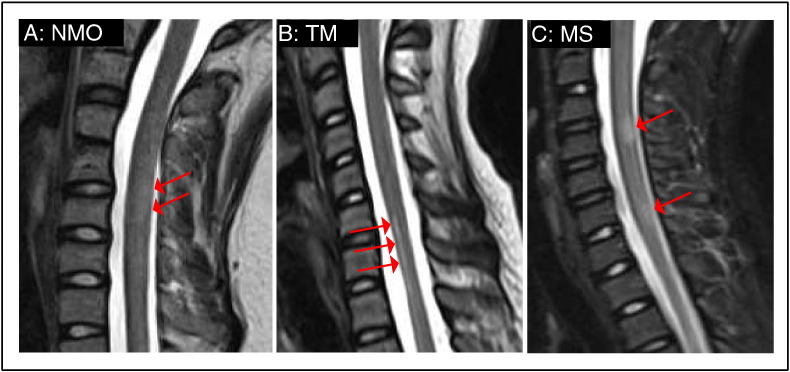
The typical pathological features of the disease process, as indicated by T2-weighted MRI (Fig. 1A–C), include cord enlargement and a focal increase in signal intensity. Some lesions may involve both transverse halves of the cord leading to the interruption of the spinal cord pathways (Choi et al., 1996). More pronounced changes in white matter than in gray matter were reported in one study (Misra et al., 1996) while another study reported both gray and white matter regions to be equally damaged in transverse myelitis (Krishnan et al., 2004).
Fig. 1.
MRI pathological features in myelitis. Sagittal T2-weighted images of (A) 13 year old female with NMO, (B) 10 year old female with transverse myelitis and (C) 10 year old female with MS. Arrows show lesion sites.
At onset, myelitis is manifested by a spectrum of symptoms including limb weakness, sensory disturbance, back pain, and bladder and bowel dysfunction. Symptoms are sudden and progress rapidly over a few hours to a few days. It has been reported that 45% of patients worsen within 24 h (Krishnan et al., 2004) and children less than 3 years old whose deficits reached maximal severity in less than 24 h of onset are less likely to regain full ambulatory ability (Verhey and Banwell, 2013; Pidcock et al., 2007; Defresne et al., 2003).
2.2. Disease prognosis and long-term consequences
Despite the unfavorable statistics on myelitis-related complications, children and young adults with this disorder show a more favorable outcome in neurological recovery compared with adults (Alper et al., 2011). Overall, 30–62% of patients with transverse myelitis have long-term neurological deficits (Alper et al., 2011) but may vary in degree. For example, 80% of children reportedly recovered to independent ambulation (Verhey and Banwell, 2013) and a study conducted on 95 children showed that 70% experienced full physical recovery and 30% had deficits in gait and bladder/bowel function (Deiva et al., 2015).
When any spinal cord disease occurs at a young age, patients are at high risk for scoliosis development (Parent et al., 2011). In a retrospective study of 130 patients injured between birth and the age of 21 years, 97% of patients injured before the growth spurt developed scoliosis compared to 52% injured after adolescent growth spurt (Dearolf et al., 1990). These numbers were further validated by Apple and associates who reported that scoliosis developed in 23% of children with SCI younger than 12 years of age compared to only 5% of adults (Apple et al., 1995). The patients studied included both traumatic and non-traumatic injuries. Longitudinal studies are needed to examine inquired scoliosis in children with myelitis.
2.3. Classification methods in spinal cord disease
2.3.1. The International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI)
Since their first edition in 1982, the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) have progressively improved and are now adopted world-wide as a method to evaluate and classify the neurological consequence of spinal cord injury (Maynard et al., 1997; Kalsi-Ryan et al., 2014). The evaluation involves strength testing of ten muscles in the upper limb and ten muscles in the lower limb, sensory testing to light touch and sharp–dull discrimination by way of pin-prick and, evaluation of deep anal pressure and contraction. Scores from the evaluation are used to classify, among other injury characteristics, the neurological, motor and sensory levels and the severity of injury (complete\incomplete). The determination of complete or incomplete is based on sensory examination at the S4–5 dermatome and results of the anorectal examination. The ISNCSCI are used in the classification of traumatic and non-traumatic SCI for all age groups and are the recommended method for evaluating acute neurological status and also for recording neurological recovery over time (Gupta et al., 2008; Waring et al., 2010; Kirshblum et al., 2011; Burns et al., 2012). There are no studies testing the validity and sensitivity of the ISNCSCI in pediatric non-traumatic SCI. Thus, the inability to reliably evaluate the consequence of myelitis using the ISNCSCI and to classify the severity of injury in children may pose considerable barriers to prognostication of recovery, definition of rehabilitation goals and evaluation of clinical trials designed for neurorepair and neurorecovery.
2.3.2. The Expanded Disability Status Scale (EDSS)
The Expanded Disability Status Scale (EDSS) was developed in 1983 and has been widely used as a primary endpoint to measure impairment in clinical trials of MS (Kurtzke, 1983). The EDSS allows clinicians to categorize and score patients' disability in eight functional systems: pyramidal, cerebral, brainstem, sensory, bowel and bladder, visual and cerebellar. Outcome measures receive scores ranging from 0.0 to 10.0 with half-point increments. In a study involving children with spinal cord inflammatory diseases the EDSS has been applied, in combination with the ISNCSCI, to identify early prognostic factors of relapse and disability (Deiva et al., 2015). In adult patients with spinal cord lesions, scores from EDSS were used to evaluate disability and monitor treatment progress in patients with NMO (Torres et al., 2015) and were shown to correlate with results from diffusion tensor imaging in patients with MS and NMO (Naismith et al., 2013).
3. Pain in myelitis
3.1. Pain prevalence
Approximately 40–80% of persons with myelitis report pain as one of the earliest symptoms (Wolf et al., 2012). In a study of 24 children with acute transverse myelitis, 88% suffered from pain and 70% of these patients reported that pain interfered with their daily physical activities and rehabilitation efforts (Defresne et al., 2003). Data from studies in both pediatric and adult populations indicate that chronic pain in childhood is predictive of pain, disability, and psychiatric disorders in adulthood (Werhagen et al., 2007; Green et al., 2012). We are unaware of any studies evaluating the temporal course of pain in children with myelitis, although, in adults, pain symptoms persist even after recovery from transverse myelitis (National Institute of Neurological Disorders and Stroke (NINDS), 2015; Defresne et al., 2003; Miyazawa et al., 2003).
3.2. Pain assessment
Pain assessment in SCI is generally performed using the International Spinal Cord Injury Pain Classification (ISCIP) system (Hjermstad et al., 2011). The classification system places SCI-related pain into four categories, namely, neuropathic, nociceptive, unknown and pain of other etiology. The predominant pain process is neuropathic pain. Neuropathic pain is defined by the International Association for the Study of Pain (IASP) as pain triggered by a lesion or disease to the somatosensory nervous system (Bryce et al., 2012). It is generally described as a burning, aching, tingling or stabbing sensation. Neuropathic pain is divided into three subtypes: at level SCI pain, below level SCI pain and other neuropathic pain. It is important to note that term level refers to the neurological level of injury defined by the ISNCSCI as the lowest (most caudal) dermatome or myotome with normal sensory and motor function. Neuropathic pain can be unilateral or bilateral and can occur in complete or incomplete injuries. In a study on the prevalence of neuropathic pain in non-traumatic SCI, 15% of the patients reported pain at injury level while 23% had below level pain (Werhagen et al., 2007). One study mentioned neuropathic pain above injury level possibly due to complex regional pain syndromes and compressive peripheral neuropathy (Sezer et al., 2015).
A number of tools are being developed and used in the screening and assessment of SCI-related pain. Despite its somewhat subjective nature, Quantitative Sensory Testing (QST) has been well investigated in patients with SCI. The test uses thermal, electrical and vibratory stimuli administered at different dermatomes to detect the pain thresholds (Savic et al., 2007; Boakye et al., 2012). The assessment of pain intensity is often performed using questionnaires and self-reported scales. The Visual Analogue Scale (VAS), Numeric Rating Scale (NRS), Leeds Assessment of Neuropathic Symptoms and Signs (LANSS), and PainDETECT questionnaire (PD-Q) provide an estimation of pain and information on pain evolution over time and the effect of treatment (Hjermstad et al., 2011; Haanpää et al., 2011; Saulino, 2014; Nakipoglu-Yuzer et al., 2013; Freynhagen et al., 2006). These pain assessment methods have been tested extensively in the adult population and in traumatic injuries. The types of pain experienced by individuals with myelitis may be caused by different underlying physiological mechanisms than traumatic injury. Given the high prevalence of pain in children with myelitis, a validated assessment and classification system for pain needs to be established in order to identify characteristic features of pain and determine suitable treatment.
3.3. Pain pathways in myelitis pathogenesis
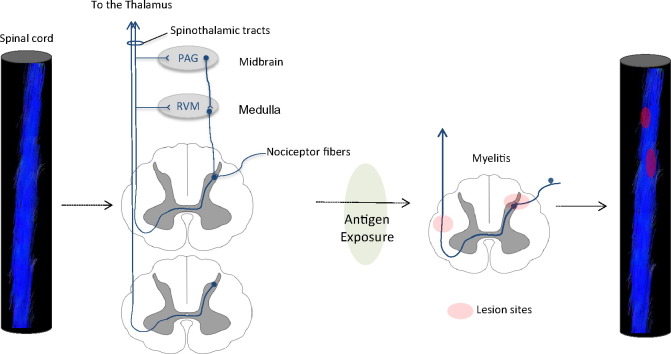
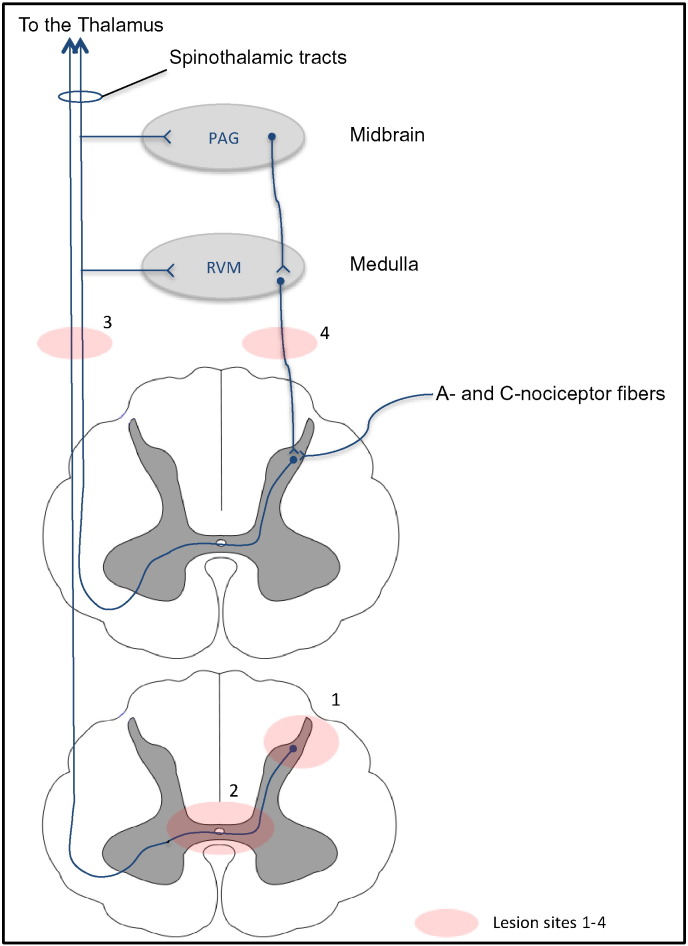
Pain sensation follows a series of mechanisms and pathways integrated from the peripheral nerves to higher cerebral structures. Pain related to transverse myelitis is poorly understood. However, pain from spinal cord injuries and pain associated with MS were reported to result from damage to any structure on the spinothalamic pathway or from demyelination of the dorsal column primary afferents (Fig. 2) (Masri and Keller, 2012; Solaro and Messmer Uccelli, 2010). The sequence of events leading to pain in patients with myelitis may start following a lesion involving the dorsal horn of the spinal cord, and subsequently alterations in the myelinated, thinly myelinated and unmyelinated axons of the A- and C-nociceptor fibers as they terminate at the spinal substantia gelatinosa (lamina II). Consequently, the injured/demyelinated afferent axons will exhibit a reduction in electrical conduction (McDonald and Sadowsky, 2002) and a change in electrophysiological properties leading to the generation of ectopic signals. Ectopic discharge has been best evaluated in the peripheral nervous system for C-fibers (Serra et al., 2012) and A-beta fibers (Devor, 2009). Such changes in electrical activity may contribute to central sensitization and the sensation of pain at the level of the brain. Pain signals are relayed through the dorsal column of the spinal cord, and ascend contralaterally to the thalamus via the spinothalamic pathway. The pain sensory signals terminate in a number of brain regions including the primary and secondary somatosensory cortices, the posterior insula cortex as well as other brain regions involved in emotional processing (Peyron et al., 2000). Several zones in the brain are implicated in pain regulation or modulation: a number of cortical and subcortical regions have outputs to descending modulatory regions in the brainstem (Villemure and Schweinhardt, 2010). The periaqueductal gray (PAG) is the primary control center for descending pain modulation (Hemington and Coulombe, 2015) and receives input from various structures in the brain. PAG neurons send descending axons to the rostral ventromedial medulla (RVM) (Odeh and Antal, 2001) and axons in the RVM project to the spinal cord dorsal horn where they depress the activity of nociceptive neurons (Schweinhardt and Bushnell, 2010). We are unaware whether these descending pathways are altered in transverse myelitis, but these changes could conceivably contribute to diminished descending modulation of pain.
Fig. 2.
Pain pathways in spinal cord demyelinating lesions. Pain sensation follows a series of mechanisms starting from peripheral nociceptor fibers to higher cerebral structures: A- and C-nociceptor fibers synapse at the spine dorsal column, pain information is relayed through the dorsal column of the spinal cord and ascends contralaterally to the thalamus via the spinothalamic pathway. A number of cortical and subcortical regions have outputs to descending pain modulation. The rostral ventromedial medulla (RVM) receives afferent input from the periaqueductal gray (PAG), axons in the RVM project to the spinal cord dorsal horn. Demyelinating lesions can affect the peripheral nerve fibers, spinal cord columns, or the ascending/descending pathways.
3.3.1. Pain mechanisms in transverse myelitis
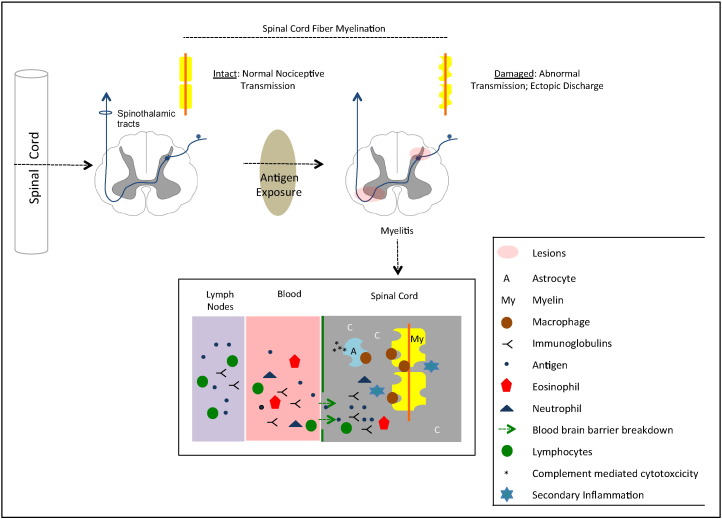
Though the mechanisms underlying lesion development and evolution in myelitis remain unknown, the current theory of pathogenesis suggests that when an infection presents in the immune system with an antigen similar to myelin, the resulting antibodies eventually attack myelin (Fig. 3). The cascade of pathological events leading to demyelination include peripheral interactions between activated lymphocytes and immunoglobulins, followed by focal monocyte and lymphocyte infiltrates into the spinal cord perivascular space. The disruption of the blood brain barrier permeability leads to a cellular influx of leukocytes (such as eosinophils and neutrophils) and an activation of astrocytes and glial cells, finally leading to local inflammation, oligodendrocyte death and axonal conducting abnormalities (Kerr and Ayetey, 2002; Krishnan et al., 2004; Bukhari et al., 2012; Wingerchuk, 2007). The inflammatory lesions can occur at the gray matter anterior horns or along the spinothalamic pathway thereby perturbing transmission of pain signals.
Fig. 3.
Schematic diagram of the pathogenesis of transverse myelitis affecting pain pathways. Top: macroscopic changes leading to altered spinal cord fiber myelination; in cases that affect the pain pathways (e.g., spinothalamic tracts), pain may be a major symptom in transverse myelitis. Bottom: microscopic changes in the spinal cord. A series of events leading to demyelination are believed to start by exposure to antigens, activation of lymphocytes and immunoglobulins, followed by eosinophils and neutrophils and astrocyte pathology (adapted from Wingerchuk, 2007 and Bukhari, 2012). Macrophages may ‘attack’ astrocytes and myelin, leading to local inflammation, oligodendrocyte death and myelin loss. Exposed axons may thus have abnormal irritability (ectopic firing) and conduction patterns.
This complex circuitry of pain mechanisms is well understood in spinal cord injuries but not in pediatric myelitis where potential restoration of function is more likely. Future research needs to identify demyelination-related pain, explore cellular interactions in the spinal cord dorsal horn, and track all the affected pathways at different disease stages. Mechanism-based research employing advanced imaging techniques and appropriate animal models will allow improved targeted pharmacological treatments and successful clinical trials.
Little is known about how central white matter changes in the spinal cord may produce neuropathic pain. Damaged white matter spinal cord tract may potentially lead to abnormal or ectopic discharges that have been associated with neuropathic pain as measured in peripheral nerves (Han et al., 2000; Yoon et al., 1996). General alterations in nerve function may include trans-synaptic changes or alterations in neurotransmitter systems. Lesions of the dorsal columns show relatively little spontaneous ectopic discharge, but activity may be enhanced by peripheral afferent traffic (Papir-Kricheli and Devor, 1988). While unknown, alterations in spinal cord axons may have enhanced activity as surmised by clinical evidence of processes such as allodynia reflecting alterations between damaged and intact neurons (Finnerup et al., 2003). We are unaware of transverse myelitis affecting the short processes of interneurons, a consideration in the potential manifestation of pain. Potential support for alterations in interneurons includes (1) that interneuronitis may contribute to motor spasms in the spinal cord in patients with acute myelitis (Brown et al., 1997); (2) in peripheral neuropathic pain, selective loss of GABAergic interneurons has been reported (Moore et al., 2002); and (3) the loss of spinal GABAergic interneurons has been reported in spinal cord injury and was associated with neuropathic pain (Meisner et al., 2010).
4. Imaging spinal cord disease
MRI has brought major contributions to the diagnosis and monitoring of myelitis. The routine imaging protocol includes spin-echo or fast spin-echo T1-weighted and T2-weighted sequences, usually in the sagittal and axial planes pre-contrast. If a lesion is observed, post-gadolinium pulse sequences are applied (Thomas and Branson, 2013). Although routine MRI is the modality of choice in the evaluation of pediatric myelitis, studies have shown it to have poor correlation with clinical status of patients with myelitis (Defresne et al., 2003; Choi et al., 1996; Hori et al., 2012; Holland, 2013). Authors report poor lesion detection in young children with mild acute myelitis and question the specificity of MRI in detecting transverse myelitis because other demyelinating disorders can mimic it by MRI criteria (Thomas and Branson, 2013; Banwell and Dale, 2015). It is suggested to conduct a follow-up scan 24–48 h later if the initial gadolinium-enhanced MRI scan is negative (Thomas et al., 2012). However, pediatric transverse myelitis has a high risk of permanent disability. Its symptoms are sudden and progress rapidly over a few hours to a few days. There is a need for timely and accurate imaging protocols with high sensitivity and specificity to discriminate between different demyelinating myelopathies.
4.1. Diffusion tensor imaging (DTI)
New neuroimaging techniques have proven successful in providing additional information about spinal cord integrity in vivo. One such technique is DTI. It quantifies diffusion of water molecules in each voxel of an image in directions parallel and transverse to the plane of neuronal axons. The unique anisotropic characteristics of the spinal cord may allow DTI to localize white matter, separate white from gray matter and assess structural damage of the cord. Fractional Anisotropy (FA), Axial Diffusivity (AD) and Radial Diffusivity (RD) are parameters derived from DTI calculations and reflect, respectively, the magnitude of anisotropy, diffusivity parallel to the spinal cord tracts and diffusivity perpendicular to the spinal cord tracts.
Since the first in-vivo DTI study of the human spinal cord in 1999, (Clark et al., 1999) various technical limitations have been reported. These are posed by the small cord volume, natural curvature, CSF pulsatile motion, respiratory and cardiac movements, susceptibility artifacts from adjacent tissue interfaces, and in pediatric imaging especially, there is the added possibility of increased subject motion. Most of these challenges are being addressed by improved receiver coils, fast imaging techniques, motion reduction during the scans, motion correction post-scans and cardiac/respiratory gating.
Studies reported the ability of DTI to detect changes in diffusion characteristics along the spinal cord and differences in these parameters between the healthy and traumatically injured spinal cord (Ellingson et al., 2008a; Ellingson et al., 2008b; Mohamed et al., 2011; Barakat et al., 2012). In the injured spinal cord, quantitative analysis of DTI parameters typically reveals a reduction in FA and an increase in diffusivity values (AD, RD and MD). These findings are generally attributed to the breakdown of the longitudinal order given by the axonal membranes and/or myelin sheaths. Anisotropic water diffusion is attributed to the ordered arrangement of myelinated axonal fiber tracts in white matter. In the case of tissue destruction where this order is affected, anisotropy is lost leading to a reduction in FA. These pathological features are not quantifiable on conventional MRI; giving DTI significant clinical value for examining the integrity of highly ordered white matter tissue. Tables 2 and 3 provide an overview on DTI parameters in the healthy and injured spinal cord in the adult and pediatric populations.
Table 2.
Quantitative imaging findings in the healthy spinal cord. WM: white matter. GM: gray matter.
| Sample characteristics |
Imaging results |
||||
|---|---|---|---|---|---|
| Reference | Sample size | Mean age (years) | Imaged levels | DTI (mean) | MTR (mean) |
| Ellingson et al., 2008a | N = 13 | 29.5 | Cervical and thoracic | FA (WM) = 0.68 FA (GM) = 0.47 MD = 0.83 × 10−3 mm2/s |
Not assessed |
| Wilm et al., 2009 | N = 4 | Not reported | Full spinal cord | FA (C5) 0.75 FA (T5) =0.69 FA (L) =0.63 |
Not assessed |
| Cheran et al., 2011 | N = 1 | 31.5 | Cervical | FA = 0.67 AD = 1.73 × 10−3 mm2/s RD = 0.49 × 10−3 mm2/s |
Not assessed |
| Cohen-Adad et al., 2011 | N = 14 | 45 | Cervical | FA = 0.6 AD = 1.68 × 10−3 mm2/s RD = 0.61 × 10−3 mm2/s |
32.2 |
| Barakat et al., 2012 | N = 25 | 13.3 | Cervical | FA = 0.5 AD = 0.97 × 10−3 mm2/s RD = 0.41 × 10−3 mm2/s |
Not assessed |
| Petersen et al. 2012 | N = 28 | 58 | Cervical | FA = 0.64 | Not assessed |
Table 3.
Quantitative imaging findings and clinical correlations in spinal cord disease.
| Sample characteristics |
Imaging results |
Clinical correlations |
||||||
|---|---|---|---|---|---|---|---|---|
| Reference | Sample size | Mean age (years) | Presentation | Imaged levels | DTI (mean) | MTR (mean) | ASIA | Pain |
| Renoux et al., 2006 | N = 15 | 42.5 | Myelitis | Cervical and thoracic | FA (lesion) = 0.59 | Not assessed | Not assessed | Not assessed |
| Lee et al., 2008 | N = 10 | 45 | Transverse myelitis | Cervical | FA (lesion) = 0.53 | Not assessed | Not assessed | Not assessed |
| Cheran et al., 2011 | N = 25 | 39.7 | Traumatic SCI | Cervical | FA = 0.49 AD = 1.28 × 10−3 mm2/s RD = 0.60 × 10−3 mm2/s |
Not assessed | Yes | Not assessed |
| Cohen-Adad et al., 2011 | N = 14 | 45 | Traumatic SCI | Cervical | FA = 0.48 AD = 1.51 × 10−3 mm2/s RD = 0.73 × 10−3 mm2/s |
26.5 | Yes | Not assessed |
| Barakat et al., 2012 | N = 1 | 11 | Transverse myelitis | Cervical | FA = 0.45 AD = 1.06 × 10−3 mm2/s RD = 0.51 × 10−3 mm2/s |
Not assessed | Yes | Not assessed |
| Petersen et al. 2012 | N = 19 | 59.7 | Traumatic SCI | Cervical | FA (C2) = 0.61 FA (C5) = 0.53 |
Not assessed | Yes | Not assessed |
| Mulcahey et al., 2013 | N = 10 | 14 | Traumatic SCI | Cervical | FA = 0.28 AD = 1.15 × 10−3 mm2/s RD = 0.80 × 10−3 mm2/s |
Not assessed | Yes | Not assessed |
Some authors describe improved resolution with small field-of-view imaging techniques (Andre et al., 2012; Wilm et al., 2009; Zaharchuk et al., 2011; Barakat et al., 2011). This technique helped in the differentiation between white and gray matter and subsequently, correlate imaging results with clinical measures at the dorsal and ventral areas of the spine. Specifically, studies indicate that FA, AD, and RD correlate strongly with motor and sensory scores from ASIA examinations (Ellingson et al., 2008; Petersen et al., 2012; Jones et al., 2013; Vedantam et al., 2015). One study reported DTI to correlate with motor scores but was not sensitive in the detection of sensory information (Cohen-Adad et al., 2011). Moreover, a study on adults with transverse myelitis reported FA to be more sensitive to detect abnormalities than T2-weighted MRI (Lee et al., 2008). Additional reports showed correlation between DTI parameters and severity of demyelination (Klawiter et al., 2011; Renoux et al., 2006) and a reduction in DTI values in regions remote from the injury site suggesting its ability to detect Wallerian axonal degeneration (Petersen et al., 2012). Additionally, DTI-based visualization methods, such as tractography, can be used for mapping white matter fiber tract trajectories (Hendrix et al., 2015). Tractography results were reported to be successful in identifying viable spinal cord fibers in locations that were normal on T2-weighted images but had abnormal FA values (Renoux et al., 2006).
4.2. Magnetization transfer imaging (MTI)
MTI is the process by which protons associated with unbound water molecules exchange their spin energy with protons bound to macromolecules (lipid content in axonal myelin) (Wolff and Balaban, 1994; Henkelman et al., 2001). When a saturation off-resonance pulse is applied, energy (magnetization) from the pool of bound molecules will move (transfer) to the pool of unbound molecules, reducing the total observed MR signal, thereby providing an indirect marker for myelin content (Schwartz and Hackney, 2003; Smith et al., 2012; Smith et al., 2014). In tissues where there is no energy exchange, the water proton signal intensity is unchanged. In tissues where there is exchange in energy, the water signal intensity decreases. A low magnetization transfer ratio (MTR) is an indicator of damage to myelin and axonal membranes.
Magnetization transfer techniques have been used in Acute Disseminated Encephalomyelitis, (Inglese et al., 2002) Amyotrophic Lateral Sclerosis (ALS) (Pradat et al., 2011), MS (Verhey and Sled, 2013; Zackowski et al., 2009; Wang et al., 2015), and demyelination in the spinal cord (Klawiter et al., 2011). Studies have also shown the possibility of using MTI to segment gray matter and white matter in the healthy adult spinal cord (Yiannakas et al., 2012) and in adults with chronic cervical SCI. MTR values were reported to predict sensory disability in dorsal columns while ventrolateral MTR values predicted motor disability (Cohen-Adad et al., 2011). The use of magnetization transfer in pediatric myelitis has not been reported.
Further work is needed to examine the feasibility and reliability of quantitative imaging biomarkers in children with demyelinating myelopathies. Advances in imaging have overcome many technical hindrances that once prevented analysis of the structural and functional properties of fiber tract. Current research evidence suggests that imaging can potentially be used to quantify tract injury at the acute, sub-acute and chronic phases of myelitis, discriminate between transverse myelitis and its mimics, identify subjects at high risk of developing further attacks and can also be used to monitor remyelination in clinical trials.
4.3. Ultrahigh field imaging: a possibility in pediatric myelitis
Rapid progress in the technical improvement of MRI systems led to the introduction of ultrahigh (>3 T) field imaging techniques. These ultrahigh field systems were developed and optimized in pursuit of the ultimate image resolution. Theoretically, signal-to-noise (SNR) ratio increases with the field strength and therefore leads to an improved diagnostic ability of MRI. Since its first introduction in the 1990s, imaging at a 7 Tesla magnetic field has gained increasing tolerability by patients and healthy volunteers (Theysohn et al., 2008; Rauschenberg et al., 2014; Ugurbil, 2014). To date, approximately 40 7 T units have been installed worldwide, although operating only in research settings (Umutlu et al., 2013; Kraff et al., 2015). In a study comparing 3 T to 7 T magnetic fields, 7 T imaging showed significantly higher SNR in the spinal cord of healthy volunteers (Sigmund et al., 2012). A later study revealed that 7 T MRI in combination with advanced coil technology can detect signal abnormality in the spinal corticospinal tract of patients with ALS (Cohen-Adad et al., 2013). Ultrahigh field imaging may be a useful imaging marker of demyelination. Since contrast is more enhanced at ultrahigh field strength, patients with demyelinating lesions may benefit from reduced gadolinium dosage (Tallantyre et al., 2011). Further work is required to investigate the utility of ultrahigh field imaging in pediatric spinal cord demyelination.
4.4. Myelin Water Fraction imaging
Alterations in myelin can be measured indirectly with MRI by applying pulse sequences to detect T2 relaxation values present in different tissue components (Laule et al., 2004). It is believed that T2 relaxation of approximately 20 ms is attributed to water contained between myelin bilayers (myelin water). The ratio of myelin water signal to total water is termed the Myelin Water Fraction (MWF) (Whittall et al., 1997). A study on measuring regional variations in myelin content along the entire spinal cord showed that MWF values were consistent with white matter and gray matter contributions in the spinal cord anatomy (Minty et al., 2009). In a repeatability study comparing MWF values in young and older adults showed a slight decrease in MWF between the two groups (MacMillan et al., 2011). In a test–retest study conducted on healthy volunteers and patients with spinal cord demyelinating lesions, high reproducibility of MWF estimates was observed in both groups, with patients showing a decrease in MWF values as compared to controls (Wu et al., 2006). Monitoring changes in myelin content is essential in studying demyelinating diseases. Myelin water measurement has the potential to identify preserved myelin and guide trials of myelin protection and repair in demyelination lesions of the spinal cord.
4.4.1. Integrating measures of spinal cord dysfunction and brain dysfunction in pain
Recent work has used functional imaging to demonstrate connectivity between rostral brain centers (Sprenger et al., 2015). A similar approach using functional white matter tractography (seeding rostral regions with known function as part of ascending pain pathways) may also be employed in patients with myelitis to map out brain regions associated with pain and other functions that are affected (Agosta et al., 2007; Oppenheim et al., 2007). Furthermore, in the future, by combining spinal cord with brain functional imaging, regions of the brain known to be affected in neuropathic pain in other conditions may be used to dissect the contribution of alterations in white matter tracts and the evolution of pain, a defined and necessary brain function (Garcia-Larrea and Peyron, 2013).
5. Conclusions
Pediatric transverse myelitis is relatively uncommon. As a result, we know very little about the distinguishing characteristics of this population. Correct diagnosis for myelitis is limited by the lack of injury classification standards specific to non-traumatic SCI, and the lack of imaging techniques to define the extent of injury and preserved neurological function. Additionally, pain represents a major problem in pediatric myelitis and an obstacle to effective rehabilitation outcomes. However despite its high prevalence, very little has been described regarding the management of chronic pain in children with inflammatory demyelinating insults to the spinal cord.
Emerging quantitative MRI techniques offer unique possibilities in examining white matter integrity and myelin content in the spinal cord. DTI and MTI can be used to assess axonal integrity and the degree of demyelination. These quantitative imaging techniques may also be useful for monitoring the efficacy of new therapeutics for myelitis. However, the many advantages of quantitative imaging are accompanied by various technical challenges. The pediatric spinal cord is small in volume and subject to susceptibility and motion artifacts that must be addressed to optimize diagnostic quality. Furthermore, for these quantitative imaging techniques to be used in clinical settings, their reliability and sensitivity have to be established in pediatric myelitis.
Acknowledgments
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) of the National Institutes of Health under award number K25HD079505 and by the National Institute of Neurological Disorders and Stroke (NINDS) under award number K24NS064050. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Agosta F., Absinta M., Sormani M.P. In vivo assessment of cervical cord damage in MS patients: a longitudinal diffusion tensor MRI study. Brain. 2007;130(8):2211–2219. doi: 10.1093/brain/awm110. 17535835 [DOI] [PubMed] [Google Scholar]
- Alper G., Petropoulou K.A., Fitz C.R., Kim Y. Idiopathic acute transverse myelitis in children: an analysis and discussion of MRI findings. Mult. Scler. 2011;17(1):74–80. doi: 10.1177/1352458510381393. 20858691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre J.B., Zaharchuk G., Saritas E. Clinical evaluation of reduced field-of-view diffusion-weighted imaging of the cervical and thoracic spine and spinal cord. A.J.N.R. Am. J. Neuroradiol. 2012;33(10):1860–1866. doi: 10.3174/ajnr.A3134. 22555576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apple D.F., Jr, Anson C.A., Hunter J.D., Bell R.B. Spinal cord injury in youth. Clin. Pediatr. (Phila) 1995;34(2):90–95. doi: 10.1177/000992289503400205. 7729113 [DOI] [PubMed] [Google Scholar]
- Banwell B., Dale R.C. Understanding risk of relapse and risk of disability after childhood transverse myelitis. Neurology. 2015;84(4):332–334. doi: 10.1212/WNL.0000000000001193. 25540313 [DOI] [PubMed] [Google Scholar]
- Banwell B., Kennedy J., Sadovnick D. Incidence of acquired demyelination of the CNS in Canadian children. Neurology. 2009;72(3):232–239. doi: 10.1212/01.wnl.0000339482.84392.bd. 19153370 [DOI] [PubMed] [Google Scholar]
- Barakat N., Hunter L., Finsterbusch J. Diffusion tensor imaging of the pediatric spinal cord using an inner-FoV EPI pulse sequence in normals and patients with SCI. Proc. Intl. Soc. Magn. Reson. Med. 2011;19 [Google Scholar]
- Barakat N., Mohamed F.B., Hunter L.N. Diffusion tensor imaging of the normal pediatric spinal cord using an inner field of view echo-planar imaging sequence. A.J.N.R. Am. J. Neuroradiol. 2012;33(6):1127–1133. doi: 10.3174/ajnr.A2924. 22300927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barakat N., Mulcahey M.J., Shah P., Samdani A., Krisa L., Faro S., Mohamed F.B. Diffusion tensor imaging in pediatric transverse myelitis: a case study. J. Pediatr. Rehabil. Med. 2012;5(4):281–286. doi: 10.3233/PRM-2012-00222. 23411769 [DOI] [PubMed] [Google Scholar]
- Boakye M., Harkema S., Ellaway P.H., Skelly A.C. Quantitative testing in spinal cord injury: overview of reliability and predictive validity. J. Neurosurg. Spine. 2012;17(1):141–150. doi: 10.3171/2012.5.AOSPINE1296. 22985380 [DOI] [PubMed] [Google Scholar]
- Brown P., Quinn N.P., Barnes D., Wren D.R., Marsden C.D. Spinal rigidity following acute myelitis. Mov. Disord. 1997;12(6):1056–1059. doi: 10.1002/mds.870120635. 9399237 [DOI] [PubMed] [Google Scholar]
- Bryce T.N., Biering-Sørensen F., Finnerup N.B. International spinal cord injury pain classification: part I. Background and description. March 6–7, 2009. Spinal Cord. 2012;50(6):413–417. doi: 10.1038/sc.2011.156. 22182852 [DOI] [PubMed] [Google Scholar]
- Bukhari W., Barnett M.H., Prain K., Broadley S.A. Molecular pathogenesis of neuromyelitis optica. Int. J. Mol. Sci. 2012;13(10):12970–12993. doi: 10.3390/ijms131012970. 23202933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns S., Biering-Sorensen F., Donovan W. International standards for neurological classification of spinal cord injury, revised 2011. Top. Spinal Cord Inj. Rehabil. 2012;18(1):85–99. doi: 10.1310/sci1801-85. 23460761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas D.D., Felix E.R. Pain after spinal cord injury: a review of classification, treatment approaches, and treatment assessment. P.M.R. 2009;1(12):1077–1090. doi: 10.1016/j.pmrj.2009.07.002. 19797006 [DOI] [PubMed] [Google Scholar]
- Cheran S., Shanmuganathan K., Zhuo J. Correlation of MR diffusion tensor imaging parameters with ASIA motor scores in hemorrhagic and nonhemorrhagic acute spinal cord injury. J. Neurotrauma. 2011;28(9):1881–1892. doi: 10.1089/neu.2010.1741. 21875333 [DOI] [PubMed] [Google Scholar]
- Chitnis T. Pediatric demyelinating diseases. CONTINUUM: Lifelong Learning in Neurology. 2013;19(4):1023–1045. doi: 10.1212/01.CON.0000433285.84973.43. [DOI] [PubMed] [Google Scholar]
- Choi K.H., Lee K.S., Chung S.O. Idiopathic transverse myelitis: MR characteristics. A.J.N.R. Am. J. Neuroradiol. 1996;17(6):1151–1160. 8791931 [PMC free article] [PubMed] [Google Scholar]
- Citterio A., Franceschini M., Spizzichino L., Reggio A., Rossi B., Stampacchia G., Gruppo Italiano Studio Epidemiologico Mielolesioni Nontraumatic spinal cord injury: an Italian survey. Arch. Phys. Med. Rehabil. 2004;85(9):1483–1487. doi: 10.1016/j.apmr.2003.09.028. 15375821 [DOI] [PubMed] [Google Scholar]
- Clark C.A., Barker G.J., Tofts P.S. Magnetic resonance diffusion imaging of the human cervical spinal cord in vivo. Magn. Reson. Med. 1999;41(6):1269–1273. doi: 10.1002/(sici)1522-2594(199906)41:6<1269::aid-mrm26>3.0.co;2-2. 10371462 [DOI] [PubMed] [Google Scholar]
- Cohen-Adad J., El Mendili M.M., Lehéricy S. Demyelination and degeneration in the injured human spinal cord detected with diffusion and magnetization transfer MRI. Neuroimage. 2011;55(3):1024–1033. doi: 10.1016/j.neuroimage.2010.11.089. 21232610 [DOI] [PubMed] [Google Scholar]
- Cohen-Adad J., Zhao W., Keil B. 7-T MRI of the spinal cord can detect lateral corticospinal tract abnormality in amyotrophic lateral sclerosis. Muscle Nerve. 2013;47(5):760–762. doi: 10.1002/mus.23720. 23553571 [DOI] [PubMed] [Google Scholar]
- Dearolf W.W., 3rd, Betz R.R., Vogel L.C., Levin J., Clancy M., Steel H.H. Scoliosis in pediatric spinal cord-injured patients. J. Pediatr. Orthop. 1990;10(2):214–218. 2312704 [PubMed] [Google Scholar]
- Defresne P., Hollenberg H., Husson B. Acute transverse myelitis in children: clinical course and prognostic factors. J. Child Neurol. 2003;18(6):401–406. doi: 10.1177/08830738030180060601. 12886975 [DOI] [PubMed] [Google Scholar]
- Deiva K., Absoud M., Hemingway C. Acute idiopathic transverse myelitis in children: early predictors of relapse and disability. Neurology. 2015;84(4):341–349. doi: 10.1212/WNL.0000000000001179. 25540303 [DOI] [PubMed] [Google Scholar]
- Devor M. Ectopic discharge in abeta afferents as a source of neuropathic pain. Exp. Brain Res. 2009;196(1):115–128. doi: 10.1007/s00221-009-1724-6. 19242687 [DOI] [PubMed] [Google Scholar]
- Ellingson B.M., Kurpad S.N., Schmit B.D. Functional correlates of diffusion tensor imaging in spinal cord injury. Biomed. Sci. Instrum. 2008;44:28–33. 19141888 [PubMed] [Google Scholar]
- Ellingson B.M., Ulmer J.L., Kurpad S.N., Schmit B.D. Diffusion tensor MR imaging of the neurologically intact human spinal cord. A.J.N.R. Am. J. Neuroradiol. 2008;29(7):1279–1284. doi: 10.3174/ajnr.A1064. 18417607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingson B.M., Ulmer J.L., Kurpad S.N., Schmit B.D. Diffusion tensor MR imaging in chronic spinal cord injury. A.J.N.R. Am. J. Neuroradiol. 2008;29(10):1976–1982. doi: 10.3174/ajnr.A1272. 18719029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnerup N.B., Johannesen I.L., Fuglsang-Frederiksen A., Bach F.W., Jensen T.S. Sensory function in spinal cord injury patients with and without central pain. Brain. 2003;126(1):57–70. doi: 10.1093/brain/awg007. 12477697 [DOI] [PubMed] [Google Scholar]
- Freynhagen R., Baron R., Gockel U., Tölle T.R. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr. Med. Res. Opin. 2006;22(10):1911–1920. doi: 10.1185/030079906X132488. 17022849 [DOI] [PubMed] [Google Scholar]
- Galvin J., Scheinberg A., New P.W. A retrospective case series of pediatric spinal cord injury and disease in Victoria, Australia. Spine. 2013;38(14):E878–E882. doi: 10.1097/BRS.0b013e318294e839. [DOI] [PubMed] [Google Scholar]
- Garcia-Larrea L., Peyron R. Pain matrices and neuropathic pain matrices: a review. Pain. 2013;154(Suppl. 1):S29–S43. doi: 10.1016/j.pain.2013.09.001. 24021862 [DOI] [PubMed] [Google Scholar]
- Green M., Berliner J., Frontera J. Pediatric idiopathic transverse myelitis presenting as an anterior cord syndrome: a case report. J. Pediatr. Rehabil. Med. 2012;5(1):1–6. doi: 10.3233/PRM-2011-0187. [DOI] [PubMed] [Google Scholar]
- Greninger A.L., Naccache S.N., Messacar K. A novel outbreak enterovirus D68 strain associated with acute flaccid myelitis cases in the USA (2012–14): a retrospective cohort study. Lancet Infect. Dis. 2015;15(6):671–682. doi: 10.1016/S1473-3099(15)70093-9. 25837569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., Taly A.B., Srivastava A., Vishal S., Murali T. Traumatic vs non-traumatic spinal cord lesions: comparison of neurological and functional outcome after in-patient rehabilitation. Spinal Cord. 2008;46(7):482–487. doi: 10.1038/sj.sc.3102168. 18227851 [DOI] [PubMed] [Google Scholar]
- Haanpää M., Attal N., Backonja M. NeuPSIG guidelines on neuropathic pain assessment. Pain. 2011;152(1):14–27. doi: 10.1016/j.pain.2010.07.031. 20851519 [DOI] [PubMed] [Google Scholar]
- Han H.C., Lee D.H., Chung J.M. Characteristics of ectopic discharges in a rat neuropathic pain model. Pain. 2000;84(2–3):253–261. doi: 10.1016/s0304-3959(99)00219-5. 10666530 [DOI] [PubMed] [Google Scholar]
- Hemington K.S., Coulombe M.A. The periaqueductal gray and descending pain modulation: why should we study them and what role do they play in chronic pain? J. Neurophysiol. 2015 doi: 10.1152/jn.00998.2014. [DOI] [PubMed] [Google Scholar]
- Hendrix P., Griessenauer C.J., Cohen-Adad J. Spinal diffusion tensor imaging: a comprehensive review with emphasis on spinal cord anatomy and clinical applications. Clin. Anat. 2015;28(1):88–95. doi: 10.1002/ca.22349. 24497009 [DOI] [PubMed] [Google Scholar]
- Henkelman R.M., Stanisz G.J., Graham S.J. Magnetization transfer in MRI: a review. N.M.R. Biomed. 2001;14(2):57–64. doi: 10.1002/nbm.683. 11320533 [DOI] [PubMed] [Google Scholar]
- Hjermstad M.J., Fayers P.M., Haugen D.F. Studies comparing numerical rating scales, verbal rating scales, and visual analogue scales for assessment of pain intensity in adults: a systematic literature review. J. Pain Symptom Manage. 2011;41(6):1073–1093. doi: 10.1016/j.jpainsymman.2010.08.016. 21621130 [DOI] [PubMed] [Google Scholar]
- Holland N.R. Acute myelopathy with normal imaging. J. Child Neurol. 2013;28(5):648–650. doi: 10.1177/0883073812448438. 22752484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori M., Fukunaga I., Masutani Y. New diffusion metrics for spondylotic myelopathy at an early clinical stage. Eur. Radiol. 2012:1797–1802. doi: 10.1007/s00330-012-2410-9. 22411307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglese M., Salvi F., Iannucci G., Mancardi G.L., Mascalchi M., Filippi M. Magnetization transfer and diffusion tensor MR imaging of acute disseminated encephalomyelitis. A.J.N.R. Am. J. Neuroradiol. 2002;23(2):267–272. 11847052 [PMC free article] [PubMed] [Google Scholar]
- Jacob A., Weinshenker B.G. An approach to the diagnosis of acute transverse myelitis. Semin. Neurol. 2008;28(1):105–120. doi: 10.1055/s-2007-1019132. 18256991 [DOI] [PubMed] [Google Scholar]
- Jones J.G., Cen S.Y., Lebel R.M., Hsieh P.C., Law M. Diffusion tensor imaging correlates with the clinical assessment of disease severity in cervical spondylotic myelopathy and predicts outcome following surgery. A.J.N.R. Am. J. Neuroradiol. 2013;34(2):471–478. doi: 10.3174/ajnr.A3199. 22821918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsi-Ryan S., Wilson J., Yang J.M., Fehlings M.G. Neurological grading in traumatic spinal cord injury. World Neurosurg. 2014;82(3–4):509–518. doi: 10.1016/j.wneu.2013.01.007. 23298673 [DOI] [PubMed] [Google Scholar]
- Kerr D.A., Ayetey H. Immunopathogenesis of acute transverse myelitis. Curr. Opin. Neurol. 2002;15(3):339–347. doi: 10.1097/00019052-200206000-00019. 12045735 [DOI] [PubMed] [Google Scholar]
- Kirshblum S.C., Burns S.P., Biering-Sorensen F. International standards for neurological classification of spinal cord injury (revised 2011) J. Spinal Cord Med. 2011;34(6):535–546. doi: 10.1179/204577211X13207446293695. 22330108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klawiter E.C., Schmidt R.E., Trinkaus K. Radial diffusivity predicts demyelination in ex vivo multiple sclerosis spinal cords. Neuroimage. 2011;55(4):1454–1460. doi: 10.1016/j.neuroimage.2011.01.007. 21238597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraff O., Fischer A., Nagel A.M., Mönninghoff C., Ladd M.E. MRI at 7 Tesla and above: demonstrated and potential capabilities. J. Magn. Reson. Imaging. 2015;41(1):13–33. doi: 10.1002/jmri.24573. 24478137 [DOI] [PubMed] [Google Scholar]
- Krishnan C., Kaplin A.I., Deshpande D.M., Pardo C.A., Kerr D.A. Transverse myelitis: pathogenesis, diagnosis and treatment. Front. Biosci. 2004;9(1):1483–1499. doi: 10.2741/1351. 14977560 [DOI] [PubMed] [Google Scholar]
- Kurtzke J.F. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurol. 1983;33(11):1444–1452. doi: 10.1212/wnl.33.11.1444. 6685237 [DOI] [PubMed] [Google Scholar]
- Laule C., Vavasour I.M., Moore G.R. Water content and myelin water fraction in multiple sclerosis. A T2 relaxation study. J. Neurol. 2004;251(3):284–293. doi: 10.1007/s00415-004-0306-6. 15015007 [DOI] [PubMed] [Google Scholar]
- Lee J.W., Park K.S., Kim J.H. Diffusion tensor imaging in idiopathic acute transverse myelitis. A.J.R. Am. J. Roentgenol. 2008;191(2):W52–W57. doi: 10.2214/AJR.07.2800. 18647886 [DOI] [PubMed] [Google Scholar]
- MacMillan E.L., Mädler B., Fichtner N. Myelin water and T(2) relaxation measurements in the healthy cervical spinal cord at 3.0 T: repeatability and changes with age. Neuroimage. 2011;54(2):1083–1090. doi: 10.1016/j.neuroimage.2010.08.076. 20832480 [DOI] [PubMed] [Google Scholar]
- Masri R., Keller A. Chronic pain following spinal cord injury. Adv. Exp. Med. Biol. 2012;760:74–88. doi: 10.1007/978-1-4614-4090-1_5. 23281514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard F.M., Jr, Bracken M.B., Creasey G. International standards for neurological and functional classification of spinal cord injury. American Spinal Injury Association. Spinal Cord. 1997;35(5):266–274. doi: 10.1038/sj.sc.3100432. 9160449 [DOI] [PubMed] [Google Scholar]
- McDonald J.W., Sadowsky C. Spinal-cord injury. The Lancet. 2002;359(9304):417–425. doi: 10.1016/S0140-6736(02)07603-1. [DOI] [PubMed] [Google Scholar]
- McKinley W.O., Seel R.T., Hardman J.T. Nontraumatic spinal cord injury: incidence, epidemiology, and functional outcome. Arch. Phys. Med. Rehabil. 1999;80(6):619–623. doi: 10.1016/s0003-9993(99)90162-4. 10378485 [DOI] [PubMed] [Google Scholar]
- Meisner J.G., Marsh A.D., Marsh D.R. Loss of GABAergic interneurons in laminae I−III of the spinal cord dorsal horn contributes to reduced GABAergic tone and neuropathic pain after spinal cord injury. J. Neurotrauma. 2010;27(4):729–737. doi: 10.1089/neu.2009.1166. 20059302 [DOI] [PubMed] [Google Scholar]
- Minty E.P., Bjarnason T.A., Laule C., MacKay A.L. Myelin water measurement in the spinal cord. Magn. Reson. Med. 2009;61(4):883–892. doi: 10.1002/mrm.21936. 19191283 [DOI] [PubMed] [Google Scholar]
- Mirand A., Peigue-Lafeuille H. Acute flaccid myelitis and enteroviruses: an ongoing story. Lancet. 2015;385(9978):1601–1602. doi: 10.1016/S0140-6736(15)60121-0. 25638661 [DOI] [PubMed] [Google Scholar]
- Misra U.K., Kalita J., Kumar S. A clinical, MRI and neurophysiological study of acute transverse myelitis. J. Neurol. Sci. 1996;138(1–2):150–156. doi: 10.1016/0022-510x(95)00353-4. 8791253 [DOI] [PubMed] [Google Scholar]
- Miyazawa R., Ikeuchi Y., Tomomasa T., Ushiku H., Ogawa T., Morikawa A. Determinants of prognosis of acute transverse myelitis in children. Pediatr. Int. 2003;45(5):512–516. doi: 10.1046/j.1442-200x.2003.01773.x. 14521523 [DOI] [PubMed] [Google Scholar]
- Mohamed F.B., Hunter L.N., Barakat N. Diffusion tensor imaging of the pediatric spinal cord at 1.5 T: preliminary results. A.J.N.R. Am. J. Neuroradiol. 2011;32(2):339–345. doi: 10.3174/ajnr.A2334. 21233227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K.A., Kohno T., Karchewski L.A., Scholz J., Baba H., Woolf C.J. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J. Neurosci. 2002;22(15):6724–6731. doi: 10.1523/JNEUROSCI.22-15-06724.2002. 12151551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcahey M.J., Samdani A.F., Gaughan J.P. Diagnostic accuracy of diffusion tensor imaging for pediatric cervical spinal cord injury. Spinal Cord. 2013;51(7):532–537. doi: 10.1038/sc.2013.36. 23608812 [DOI] [PubMed] [Google Scholar]
- Nair K.P., Taly A.B., Maheshwarappa B.M., Kumar J., Murali T., Rao S. Nontraumatic spinal cord lesions: a prospective study of medical complications during in-patient rehabilitation. Spinal Cord. 2005;43(9):558–564. doi: 10.1038/sj.sc.3101752. 15824754 [DOI] [PubMed] [Google Scholar]
- Naismith R.T., Xu J., Klawiter E.C. Spinal cord tract diffusion tensor imaging reveals disability substrate in demyelinating disease. Neurology. 2013;80(24):2201–2209. doi: 10.1212/WNL.0b013e318296e8f1. 23667060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakipoglu-Yuzer G.F., Atçı N., Ozgirgin N. Neuropathic pain in spinal cord injury. Pain Physician. 2013;16(3):259–264. 23703412 [PubMed] [Google Scholar]
- National Institute of Neurological Disorders and Stroke (NINDS) updated 2015. Transverse Myelitis Fact Sheet. http://www.ninds.nih.gov/disorders/transversemyelitis/detail_transversemyelitis.htm. [Google Scholar]
- New P.W. Functional outcomes and disability after nontraumatic spinal cord injury rehabilitation: results from a retrospective study. Arch. Phys. Med. Rehabil. 2005;86(2):250–261. doi: 10.1016/j.apmr.2004.04.028. [DOI] [PubMed] [Google Scholar]
- Odeh F., Antal M. The projections of the midbrain periaqueductal grey to the pons and medulla oblongata in rats. Eur. J. Neurosci. 2001;14(8):1275–1286. doi: 10.1046/j.0953-816x.2001.01760.x. 11703456 [DOI] [PubMed] [Google Scholar]
- Oppenheim C., Ducreux D., Rodrigo S. Diffusion tensor imaging and tractography of the brain and spinal cord. J. Radiol. 2007;88(3 Pt 2):510–520. doi: 10.1016/s0221-0363(07)89850-7. 17457261 [DOI] [PubMed] [Google Scholar]
- Papir-Kricheli D., Devor M. Abnormal impulse discharge in primary afferent axons injured in the peripheral versus the central nervous system. Somatosens. Mot. Res. 1988;6(1):63–77. doi: 10.3109/08990228809144641. 2853900 [DOI] [PubMed] [Google Scholar]
- Parent S., Mac-Thiong J.M., Roy-Beaudry M., Sosa J.F., Labelle H. Spinal cord injury in the pediatric population: a systematic review of the literature. J. Neurotrauma. 2011;28(8):1515–1524. doi: 10.1089/neu.2009.1153. 21501096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen J.A., Wilm B.J., von Meyenburg J. Chronic cervical spinal cord injury: DTI correlates with clinical and electrophysiological measures. J. Neurotrauma. 2012;29(8):1556–1566. doi: 10.1089/neu.2011.2027. 22150011 [DOI] [PubMed] [Google Scholar]
- Peyron R., Laurent B., Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis. Neurophysiol. Clin. 2000;2000(30 (5)):263–288. doi: 10.1016/s0987-7053(00)00227-6. [pii: S0987-7053(00)00227-6] [DOI] [PubMed] [Google Scholar]
- Pfeiffer H.C., Bragstad K., Skram M.K. Two cases of acute severe flaccid myelitis associated with enterovirus D68 infection in children, Norway, autumn 2014. Euro Surveill. 2015;20(10):21062. doi: 10.2807/1560-7917.es2015.20.10.21062. 25788251 [DOI] [PubMed] [Google Scholar]
- Pidcock F.S., Krishnan C., Crawford T.O., Salorio C.F., Trovato M., Kerr D.A. Acute transverse myelitis in childhood: center-based analysis of 47 cases. Neurol. 2007;68(18):1474–1480. doi: 10.1212/01.wnl.0000260609.11357.6f. 17470749 [DOI] [PubMed] [Google Scholar]
- Pittock S.J., Lucchinetti C.F. Inflammatory transverse myelitis: evolving concepts. Curr. Opin. Neurol. 2006;19(4):362–368. doi: 10.1097/01.wco.0000236615.59215.d3. 16914974 [DOI] [PubMed] [Google Scholar]
- Pradat P., Cohen-Adad J., El Mendili M. Diffusion and magnetization transfer imaging detects spinal cord lesions in amyotrophic lateral sclerosis. Proc. Intl. Soc. Magn. Reson. Med. 2011;19:2465. [Google Scholar]
- Rauschenberg J., Nagel A.M., Ladd S.C. Multicenter study of subjective acceptance during magnetic resonance imaging at 7 and 9.4 T. Invest. Radiol. 2014;49(5):249–259. doi: 10.1097/RLI.0000000000000035. 24637589 [DOI] [PubMed] [Google Scholar]
- Renoux J., Facon D., Fillard P., Huynh I., Lasjaunias P., Ducreux D. MR diffusion tensor imaging and fiber tracking in inflammatory diseases of the spinal cord. A.J.N.R. Am. J. Neuroradiol. 2006;27(9):1947–1951. 17032873 [PMC free article] [PubMed] [Google Scholar]
- Saulino M. Spinal cord injury pain. Phys. Med. Rehabil. Clin. N. Am. 2014;25(2):397–410. doi: 10.1016/j.pmr.2014.01.002. 24787340 [DOI] [PubMed] [Google Scholar]
- Savic G., Bergström E.M., Davey N.J. Quantitative sensory tests (perceptual thresholds) in patients with spinal cord injury. J. Rehabil. Res. Dev. 2007;44(1):77–82. doi: 10.1682/jrrd.2005.08.0137. 17551861 [DOI] [PubMed] [Google Scholar]
- Schomberg D., Ahmed M., Miranpuri G., Olson J., Resnick D.K. Neuropathic pain: role of inflammation, immune response, and ion channel activity in central injury mechanisms. Ann. Neurosci. 2012;19(3):125–132. doi: 10.5214/ans.0972.7531.190309. 25205985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E.D., Hackney D.B. Diffusion-weighted MRI and the evaluation of spinal cord axonal integrity following injury and treatment. Exp. Neurol. 2003;184(2):570–589. doi: 10.1016/S0014-4886(03)00295-4. 14769351 [DOI] [PubMed] [Google Scholar]
- Schweinhardt P., Bushnell M.C. Pain imaging in health and disease — how far have we come? J. Clin. Invest. 2010;120(11):3788–3797. doi: 10.1172/JCI43498. 21041961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scivoletto G., Morganti B., Ditunno P., Ditunno J.F., Molinari M. Effects on age on spinal cord lesion patients' rehabilitation. Spinal Cord. 2003;41(8):457–464. doi: 10.1038/sj.sc.3101489. 12883544 [DOI] [PubMed] [Google Scholar]
- Serra J., Bostock H., Solà R. Microneurographic identification of spontaneous activity in C-nociceptors in neuropathic pain states in humans and rats. Pain. 2012;153(1):42–55. doi: 10.1016/j.pain.2011.08.015. 21993185 [DOI] [PubMed] [Google Scholar]
- Sezer N., Akkuş S., Uğurlu F.G. Chronic complications of spinal cord injury. World J. Orthop. 2015;6(1):24–33. doi: 10.5312/wjo.v6.i1.24. 25621208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddall P.J., Loeser J.D. Pain following spinal cord injury. Spinal Cord. 2001;39(2):63–73. doi: 10.1038/sj.sc.3101116. 11402361 [DOI] [PubMed] [Google Scholar]
- Siddall P.J., Taylor D.A., Cousins M.J. Classification of pain following spinal cord injury. Spinal Cord. 1997;35(2):69–75. doi: 10.1038/sj.sc.3100365. 9044512 [DOI] [PubMed] [Google Scholar]
- Sigmund E.E., Suero G.A., Hu C. High-resolution human cervical spinal cord imaging at 7 T. N.M.R. Biomed. 2012;25(7):891–899. doi: 10.1002/nbm.1809. 22183956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A.K., Dortch R.D., Dethrage L.M., Smith S.A. Rapid, high-resolution quantitative magnetization transfer MRI of the human spinal cord. Neuroimage. 2014;95:106–116. doi: 10.1016/j.neuroimage.2014.03.005. 24632465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.A., Pekar J.J., van Zijl P.C. Advanced MRI strategies for assessing spinal cord injury. Handbook Clin. Neurol. 2012;109:85–101. doi: 10.1016/B978-0-444-52137-8.00006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaro C., Messmer Uccelli M. Pharmacological management of pain in patients with multiple sclerosis. Drugs. 2010;70(10):1245–1254. doi: 10.2165/11537930-000000000-00000. 20568832 [DOI] [PubMed] [Google Scholar]
- Sprenger C., Finsterbusch J., Büchel C. Spinal cord–midbrain functional connectivity is related to perceived pain intensity: a combined spino-cortical FMRI study. J. Neurosci. 2015;35(10):4248–4257. doi: 10.1523/JNEUROSCI.4897-14.2015. 25762671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallantyre E.C., Dixon J.E., Donaldson I. Ultra-high-field imaging distinguishes MS lesions from asymptomatic white matter lesions. Neurology. 2011;76(6):534–539. doi: 10.1212/WNL.0b013e31820b7630. 21300968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theysohn J.M., Maderwald S., Kraff O., Moenninghoff C., Ladd M.E., Ladd S.C. Subjective acceptance of 7 Tesla MRI for human imaging. Magma. 2008;21(1–2):63–72. doi: 10.1007/s10334-007-0095-x. 18064501 [DOI] [PubMed] [Google Scholar]
- Thomas T., Branson H.M. Childhood transverse myelitis and its mimics. Neuroimaging Clin. N. Am. 2013;23(2):267–278. doi: 10.1016/j.nic.2012.12.006. 23608689 [DOI] [PubMed] [Google Scholar]
- Thomas T., Branson H.M., Verhey L.H. The demographic, clinical, and magnetic resonance imaging (MRI) features of transverse myelitis in children. J. Child Neurol. 2012;27(1):11–21. doi: 10.1177/0883073811420495. 21968984 [DOI] [PubMed] [Google Scholar]
- Torres J., Pruitt A., Balcer L., Galetta S., Markowitz C., Dahodwala N. Analysis of the treatment of neuromyelitis optica. J. Neurol. Sci. 2015;351(1–2):31–35. doi: 10.1016/j.jns.2015.02.012. 25727350 [DOI] [PubMed] [Google Scholar]
- Transverse Myelitis Consortium Working Group Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurol. 2002;59(4):499–505. doi: 10.1212/wnl.59.4.499. 12236201 [DOI] [PubMed] [Google Scholar]
- Ugurbil K. Magnetic resonance imaging at ultrahigh fields. I.E.E.E. Trans. Biomed. Eng. 2014;61(5):1364–1379. doi: 10.1109/TBME.2014.2313619. 24686229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umutlu L., Bitz A.K., Maderwald S. Contrast-enhanced ultra-high-field liver MRI: a feasibility trial. Eur. J. Radiol. 2013;82(5):760–767. doi: 10.1016/j.ejrad.2011.07.004. 21862273 [DOI] [PubMed] [Google Scholar]
- Vedantam A., Eckardt G., Wang M.C., Schmit B.D., Kurpad S.N. Clinical correlates of high cervical fractional anisotropy in acute cervical spinal cord injury. World Neurosurg. 2015;83(5):824–828. doi: 10.1016/j.wneu.2013.09.017. 24055569 [DOI] [PubMed] [Google Scholar]
- Verhey L.H., Banwell B.L. Inflammatory, vascular, and infectious myelopathies in children. Handb. Clin. Neurol. 2013;112:999–1017. doi: 10.1016/B978-0-444-52910-7.00020-9. [DOI] [PubMed] [Google Scholar]
- Verhey L.H., Sled J.G. Advanced magnetic resonance imaging in pediatric multiple sclerosis. Neuroimaging Clin. N. Am. 2013;23(2):337–354. doi: 10.1016/j.nic.2012.12.011. 23608694 [DOI] [PubMed] [Google Scholar]
- Villemure C., Schweinhardt P. Supraspinal pain processing: distinct roles of emotion and attention. Neuroscientist. 2010;16(3):276–284. doi: 10.1177/1073858409359200. 20360603 [DOI] [PubMed] [Google Scholar]
- Wang Y., Sun P., Wang Q. Differentiation and quantification of inflammation, demyelination and axon injury or loss in multiple sclerosis. Brain. 2015;138(5):1223–1238. doi: 10.1093/brain/awv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring W.P., Biering-Sorensen F., Burns S. 2009 review and revisions of the international standards for the neurological classification of spinal cord injury. J. Spinal Cord Med. 2010;33(4):346–352. doi: 10.1080/10790268.2010.11689712. 21061894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werhagen L., Hultling C., Molander C. The prevalence of neuropathic pain after non-traumatic spinal cord lesion. Spinal Cord. 2007;45(9):609–615. doi: 10.1038/sj.sc.3102000. 17160075 [DOI] [PubMed] [Google Scholar]
- Whittall K.P., MacKay A.L., Graeb D.A., Nugent R.A., Li D.K., Paty D.W. In vivo measurement of T2 distributions and water contents in normal human brain. Magn. Reson. Med. 1997;37(1):34–43. doi: 10.1002/mrm.1910370107. 8978630 [DOI] [PubMed] [Google Scholar]
- Wilm B.J., Gamper U., Henning A., Pruessmann K.P., Kollias S.S., Boesiger P. Diffusion-weighted imaging of the entire spinal cord. NMR Biomed. 2009;22(2):174–181. doi: 10.1002/nbm.1298. PMID: 18727164. [DOI] [PubMed] [Google Scholar]
- Wingerchuk D.M. Diagnosis and treatment of neuromyelitis optica. Neurologist. 2007;13(1):2–11. doi: 10.1097/01.nrl.0000250927.21903.f8. 17215722 [DOI] [PubMed] [Google Scholar]
- Wolf V.L., Lupo P.J., Lotze T.E. Pediatric acute transverse myelitis overview and differential diagnosis. J. Child Neurol. 2012;27(11):1426–1436. doi: 10.1177/0883073812452916. 22914370 [DOI] [PubMed] [Google Scholar]
- Wolff S.D., Balaban R.S. Magnetization transfer imaging: practical aspects and clinical applications. Radiology. 1994;192(3):593–599. doi: 10.1148/radiology.192.3.8058919. 8058919 [DOI] [PubMed] [Google Scholar]
- Wu Y., Alexander A.L., Fleming J.O., Duncan I.D., Field A.S. Myelin water fraction in human cervical spinal cord in vivo. J. Comput. Assist. Tomogr. 2006;30(2):304–306. doi: 10.1097/00004728-200603000-00026. 16628052 [DOI] [PubMed] [Google Scholar]
- Yezierski R.P. Pain following spinal cord injury: the clinical problem and experimental studies. Pain. 1996;68(2–3):185–194. doi: 10.1016/s0304-3959(96)03178-8. 9121805 [DOI] [PubMed] [Google Scholar]
- Yiannakas M.C., Kearney H., Samson R.S. Feasibility of grey matter and white matter segmentation of the upper cervical cord in vivo: a pilot study with application to magnetisation transfer measurements. Neuroimage. 2012;63(3):1054–1059. doi: 10.1016/j.neuroimage.2012.07.048. 22850571 [DOI] [PubMed] [Google Scholar]
- Yoon Y.W., Na H.S., Chung J.M. Contributions of injured and intact afferents to neuropathic pain in an experimental rat model. Pain. 1996;64(1):27–36. doi: 10.1016/0304-3959(95)00096-8. 8867245 [DOI] [PubMed] [Google Scholar]
- Zackowski K.M., Smith S.A., Reich D.S. Sensorimotor dysfunction in multiple sclerosis and column-specific magnetization transfer-imaging abnormalities in the spinal cord. Brain. 2009;132(5):1200–1209. doi: 10.1093/brain/awp032. 19297508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaharchuk G., Saritas E.U., Andre J.B. Reduced field-of-view diffusion imaging of the human spinal cord: comparison with conventional single-shot echo-planar imaging. A.J.N.R. Am. J. Neuroradiol. 2011;32(5):813–820. doi: 10.3174/ajnr.A2418. 21454408 [DOI] [PMC free article] [PubMed] [Google Scholar]