Abstract
Development of the human pancreas is well-known to involve tightly controlled differentiation of pancreatic precursors to mature cells that express endocrine- or exocrine-specific protein products. However, details of human pancreatic development at the ultrastructural level are limited. The present study analyzed 8–20 week fetal age human pancreata using scanning and transmission electron microscopy (TEM), TEM immunogold and double or triple immunofluorescence staining. Primary organization of islets and acini occurred during the developmental period examined. Differentiating endocrine and exocrine cells developed from the ductal tubules and subsequently formed isolated small clusters. Extracellular matrix fibers and proteins accumulated around newly differentiated cells during their migration and cluster formation. Glycogen expression was robust in ductal cells of the pancreas from 8–15 weeks of fetal age; however, this became markedly reduced at 20 weeks, with a concomitant increase in acinar cell glycogen content. Insulin secretory granules transformed from being dense and round at 8 weeks to distinct geometric (multilobular, crystalline) structures by 14–20 weeks. Initially many of the differentiating endocrine cells were multihormonal and contained polyhormonal granules; by 20 weeks, monohormonal cells were in the majority. Interestingly, certain secretory granules in the early human fetal pancreatic cells showed positivity for both exocrine (amylase) and endocrine proteins. This combined ultrastructural and immunohistochemical study showed that, during early developmental stages, the human pancreas contains differentiating epithelial cells that associate closely with the extracellular matrix, have dynamic glycogen expression patterns and contain polyhormonal as well as mixed endocrine/exocrine granules.
Keywords: electron microscopy, glycogen, human fetal pancreas, insulin granules, multihormonal cells, polyhormonal granules
Abbreviations
- SEM
scanning electron microscopy
- TEM
transmission electron microscopy
- CK19
Cytokeratin 19
- ECM
extracellular matrix
- PDX-1
pancreatic and duodenal homeobox 1
Introduction
The pancreas is a gastrointestinal-derived organ composed of endocrine and exocrine compartments. Development of the human pancreas begins on day 26 post-conception when the dorsal pancreatic bud evaginates followed by ventral bud formation from the gut tube.1 Many factors are involved in these processes, including interactions with the mesenchyme2 and expression of pancreatic-specific transcription factors.3,4 Both buds then elongate and branch extensively, followed by gut tube rotation, bud fusion and duct interconnection by the 6th or 7th week fetal age.1,5 By 7.5 weeks, insulin-positive cells are detectable in the fetal pancreas; glucagon and somatostatin are present by 8.5 weeks.1 Pancreatic polypeptide-expressing cells follow, with expression at 10 weeks, in conjunction with an overall increase in hormone-expressing cells.1
Up to ∼9 weeks of fetal life, a majority of the hormone-expressing cells are isolated or in small aggregates located adjacent to the ductal tree6 and often co-express cytokeratin 19 (CK19; a marker of ductal cells), suggesting that ductal cells represent a population of pancreatic progenitors.1,4,7 By 10–12 weeks, insulin-positive cells begin to cluster with glucagon-positive cells. By 13–15 weeks, these cells, along with other hormone-expressing cells, aggregate into larger immature islet structures that are beginning to be invaded by blood vessels.1,4,7 The close association of endocrine cells with ducts has been observed not only during human pancreatic development8 but also in the adult human pancreas.9 Pancreatic ducts, identified by carbonic anhydrase II, were shown to be progenitors for developing islets.10
Ultrastructural analysis provides insight into cellular compartments, including organelles and secretory granules, along with general cellular morphology. Transmission electron microscopy analysis of human fetal pancreatic exocrine tissue has shown increases in rough endoplasmic reticulum and zymogen granules from 12 to 19 weeks fetal age.11 Similar investigations of the human fetal endocrine compartment at 12–20 weeks fetal age have identified, by immunohistochemistry, numerous epithelial cells that not only contained multiple pancreatic endocrine hormones12,13 but also individual granules containing multiple hormones, as analyzed by electron microscopy.14,15 However, details of human pancreatic development at the ultrastructural level are limited. The objective of the present study was to analyze human pancreatic ultrastructure from 8 to 20 weeks of fetal life using a 3-pronged approach: scanning and transmission electron microscopy (SEM and TEM), TEM immunogold staining and double as well as triple immunofluorescence staining.
Materials and Methods
Human fetal pancreas collection
Human fetal pancreatic samples (8–20 weeks fetal age) were collected according to protocols approved by the Health Sciences Research Ethics Board at Western University, in line with the Canadian Council on Health Sciences Research Involving Human Subjects guidelines. Samples were dissected immediately and processed for electron microscope, immunogold and immunofluorescence analyses.
Tissue processing for transmission electron microscopy
Fetal pancreata were fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer and post-fixed with 1% osmium tetroxide. After dehydration through a graded ethanol series and propylene oxide treatment, samples were embedded in araldite medium and cured at 65°C overnight. Semi-thin (500 nm) and ultra-thin (60 nm) sections were prepared. Silver-gold ultra-thin sections were stained with 2% uranyl acetate in ethanol and Reynolds’ lead citrate, then examined with a Philips 410 electron microscope (FEI, Hillsboro, OR, USA) at 60 kgvolts. Eight pancreata collected from 8 to 20 weeks of fetal age were processed for TEM analysis. Cells or clusters labeled as “Endo” are identified by the presence of endocrine granule structures.
Tissue processing for scanning electron microscopy
Human fetal pancreas samples were cut into 2 × 2 mm fragments, fixed with 2.5% glutaraldehyde at 4°C overnight and then dehydrated with an increasing concentration gradient of ethanol. Samples were subjected to critical point drying and palladium-gold coating, then visualization by a 3400-N Variable Pressure Scanning Electron Microscope (Hitachi, Saskatoon, SK, Canada). Four pancreata collected from 9, 14, 16 and 20 weeks of fetal age were processed for SEM analysis.
Immunogold labeling for transmission electron microscopy
Processed human fetal ultra-thin sections cut at a thickness of 60 nm were adhered to formvar-carbon coated nickel grids (Canemco-Marivac, Gore, QC, Canada). After blocking non-specific binding sites, grids were treated with primary antibodies: insulin (I2018, Sigma), glucagon (sc-13091, Santa Cruz), somatostatin (18-0078), pancreatic polypeptide (18-0043, Zymed) and amylase (171534, Calbiochem). Grids were then incubated with a secondary antibody conjugated with either 10 nm or 20 nm gold particles (ab27241 and ab27237, Abcam) and counterstained with 2% uranyl acetate in water. Staining was reviewed with a Philips 410 electron microscope (FEI, Hillsboro, OR, USA) at 60 kgvolts. Negative controls omitted the primary or secondary antibodies.
Immunofluorescence and PAS staining
Human fetal pancreas samples were fixed in 4% paraformaldehyde and paraffin embedded. Sections (4 μm) were prepared and stained with either appropriate dilutions of primary antibodies, including CK19 (M0888, Dako), pancreatic and duodenal homeobox 1 (PDX-1; Dr. Wright, University of Vanderbilt), insulin (18-0067, Invitrogen, Camarillo, CA, USA), glucagon (G2654, Sigma), somatostatin (ab30788, Abcam), pancreatic polypeptide (18-0043, Zymed), amylase (171534, Calbiochem), collagen I (ab6308, Abcam), collagen IV (MAB1910), laminin (AB19012) and fibronectin (MAB1926, Millipore) or Schiff's reagent for PAS staining (24200-1, Polysciences Inc.).16 Slides were then treated with either fluorescent secondary antibodies (obtained from Jackson Immunoresearch Laboratories) or haematoxylin as a counterstain, and at least 3 pancreata per age group were examined.
Results
Histological and ultrastructural changes in the 8–20 week human fetal pancreas
Double immunofluorescence staining for insulin/glucagon in the human fetal pancreas showed increased endocrine cell presence and organization into islet-like structures as development occurred (Fig. 1A). As well, multihormonal insulin/glucagon double positive cells decreased as the pancreas aged. Staining for CK19/amylase revealed a switch between high ductal presence/few acinar cells at 8 weeks, to fewer ducts/many acinar cells at 20 weeks (Fig. 1A). Initially, at 8 weeks, pancreatic epithelial cells were organized into ductal structures surrounded by scattered individual mesenchymal-like stellate cells; multiple layers of the epithelial cells with few or no granules suggest outgrowth from the ducts (Fig. 1B/C, left panels). Also, at 8 weeks, CK19 and amylase co-localization was detected in some of these cells (Fig. 1A, lower panel). By 14 weeks, epithelial cells had formed small clusters separate from the ducts (Fig. 1B/C, middle panels). From 14–20 weeks, acinar cells increased in number and formed amylase+ cell clusters with centroacinar cells labeled by CK19 (Fig. 1A, middle and right panels). Other cell clusters that stained positive for insulin and glucagon had increased in numbers and formed more developmentally islet-like structure that contained capillaries from 14–20 weeks of age (Fig. 1A/B/C, middle and right panels).
Figure 1.
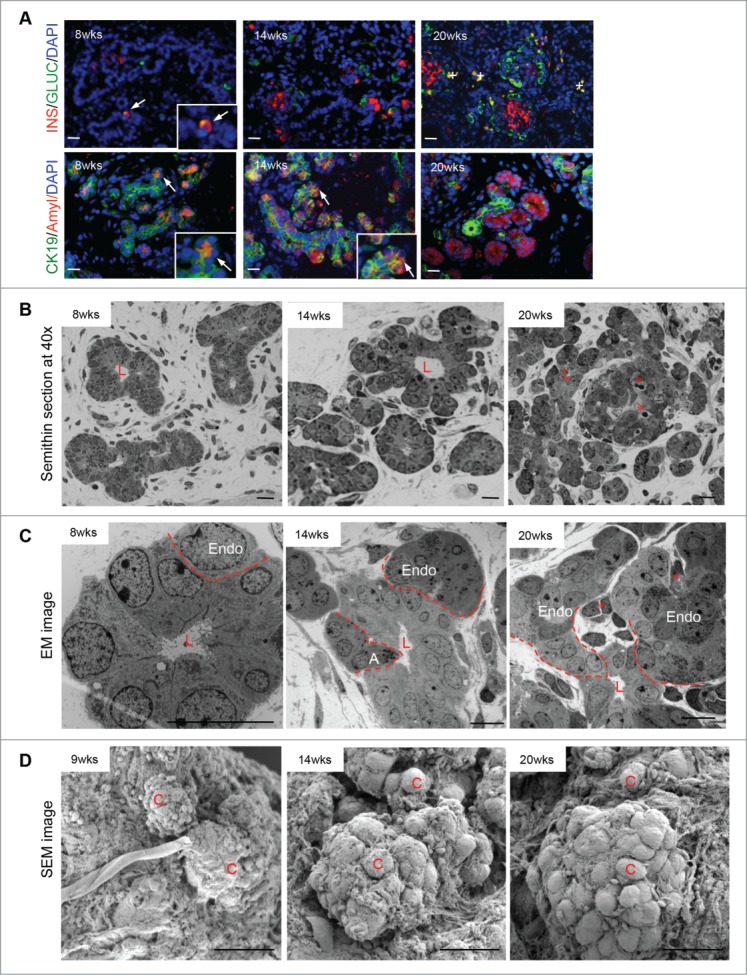
The developing (8–20 week) human fetal pancreas. (A) Double immunofluorescence for glucagon or CK19 (green) with insulin or amylase (red) and DAPI stained nuclei in blue. Arrows indicate double positive labeled cells and enlarged images inserted, while + labels red blood cells, scale bar = 50 μm. Representative images from at least 3 pancreata per age group are shown. (B) Semithin, (C) transmission electron and (D) scanning electron micrographs of human fetal pancreata. Representative images from 4–8 pancreata are shown. A = acinar cell; C = cell, Endo = endocrine cells, L = lumen of duct, asterisks = blood vessels, and the dashed line outline newly differentiated cells emerging from the duct. Scale bars for semithin and scanning electron micrographs (SEM) = 50 μm, transmission electron micrographs (TEM) = 10 μm.
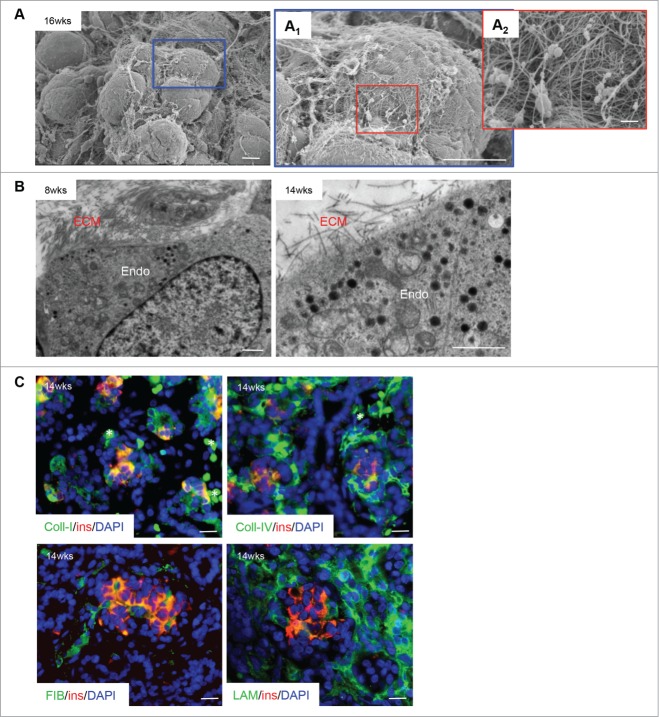
SEM images demonstrated a significant amount of extracellular matrix (ECM) fibers and small scattered cells at 9 weeks; by 14–20 weeks there was a major increase in the number of epithelial cell clusters with a complex intercalation of the ECM (Fig. 1D). Higher magnification of the SEM images showed a pronounced ECM presence around the cell clusters as well as surrounding each cell (Fig. 2A/A1/A2, B). To confirm the presence of ECM proteins, double immunofluorescence was performed: 4 ECM proteins (collagen I and IV, fibronectin and laminin) were ubiquitously expressed in the pancreas, especially within endocrine cells and surrounding endocrine cell clusters (Fig. 2C).
Figure 2.
Extracellular matrix localization in the developing human fetal pancreas. (A) Scanning electron micrographs of a human fetal pancreatic cluster at 16 weeks fetal age indicating a complex organization of extracellular matrix (ECM) on the cell surface. Highlighted areas are enlarged in (A1) and (A2). Scale bars = 10 μm (A and A1) and 1 μm (A2). (B) Transmission electron micrograph of ECM-endocrine cell interactions in the human fetal pancreas (8 and 14 weeks fetal age). Endo = endocrine cells, scale bars = 1 μm. (C) Double immunofluorescence staining of a 14 week fetal age pancreas for insulin (red) with collagen I, collagen IV, fibronectin or laminin (all green), and DAPI-stained nuclei in blue. Scale bar = 50 μm, asterisks = blood cells. Representative images from 4–10 pancreata are shown.
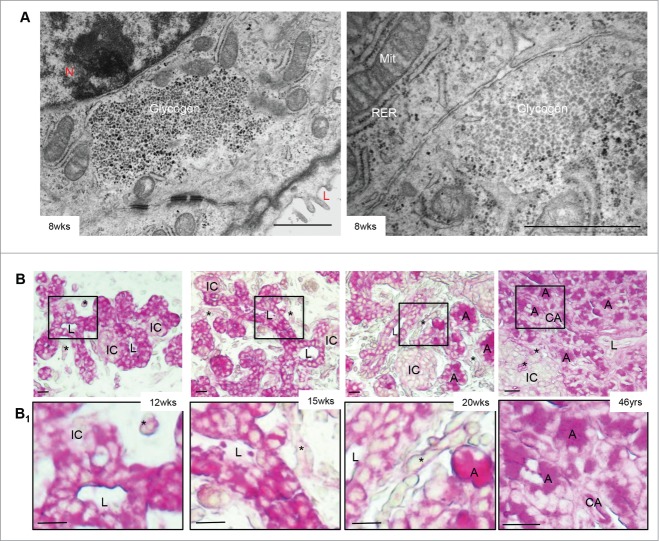
Another interesting observation was of glycogen accumulation in the human fetal pancreas (Fig. 3A/B). Glycogen-positivity, determined by PAS staining, was very strong in ductal cells until 15 weeks of fetal life with light staining in the newly formed endocrine cell clusters (Fig. 3B/B1, left and 2nd panels), similar to what has been previously reported.6 By 20 weeks, however, glycogen expression in ductal cells was reduced, while pancreatic exocrine cell clusters became increasingly more positive (Fig. 3B/B1, 3rd panels). In the adult pancreas, the glycogen staining was observed in the exocrine compartment with no staining in endocrine or ductal cells, in accordance with previous reports (Fig. 3B/B1, right panels).6
Figure 3.
Glycogen expression in the developing human fetal pancreas. (A) Transmission electron micrographs of the 8 week human fetal pancreas showed glycogen positivity in ductal cells. Scale bars = 1 μm. L = lumen, Mit = mitochondria, N = nuclei, RER = rough endoplasmic reticulum. (B) PAS stain (pink) indicates glycogen, which is highly expressed in ducts between 12–15 weeks. By 20 weeks of fetal age, glycogen expression is reduced in ductal cells and is expressed primarily in surrounding acinar cell clusters with no islet or ductal cell expression in the adult pancreas. Highlighted areas are enlarged in B1. Scale bar = 50 μm. Representative PAS images from at least 3 pancreata per age group are shown. A = acinar; CA = centroacinar; IC = endocrine cell cluster; L = lumen of duct; asterisks = blood vessels.
Human insulin granules display distinct geometric structures
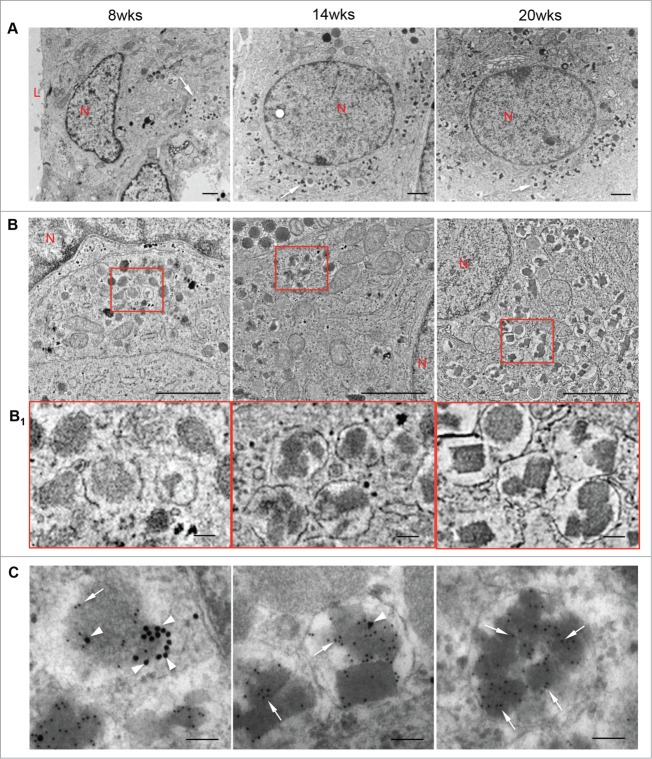
At 8 weeks fetal age, newly developed β-cells contained only a few insulin granules that were dense and round (Fig. 4A/B/B1, left panels) and frequently co-expressed glucagon, as determined by double immunogold staining (Fig. 4C, left panel). From 14 to 20 weeks, many of the insulin granules developed a distinct geometric (multilobular, crystalline) morphology, which represents mature insulin granules (Fig. 4, middle and right panels). By 20 weeks, crystalline insulin granules were the predominant form observed while there was a marked decrease in the number of polyhormonal granules in the β-cells (Fig. 4, right panels). Other types of pancreatic endocrine granules, identified by immunogold labeling, showed relatively round granule structures (Figs. 5 and 6).
Figures 4.
Insulin granules in the developing human fetal pancreas. Transmission electron micrographs of β-cells (A), insulin granules in β-cells (B) with highlighted areas enlarged in (B1); and double immunogold staining for insulin (10 nm, arrows) and glucagon (20 nm, arrowheads) (C) in 8, 14 and 20 week human fetal pancreata. Representative images from 8 pancreata are shown. L = lumen, N = nuclei. Scale bars = 1 μm (A, B) and 100 nm (B1, C). Arrows in A indicate insulin granules.
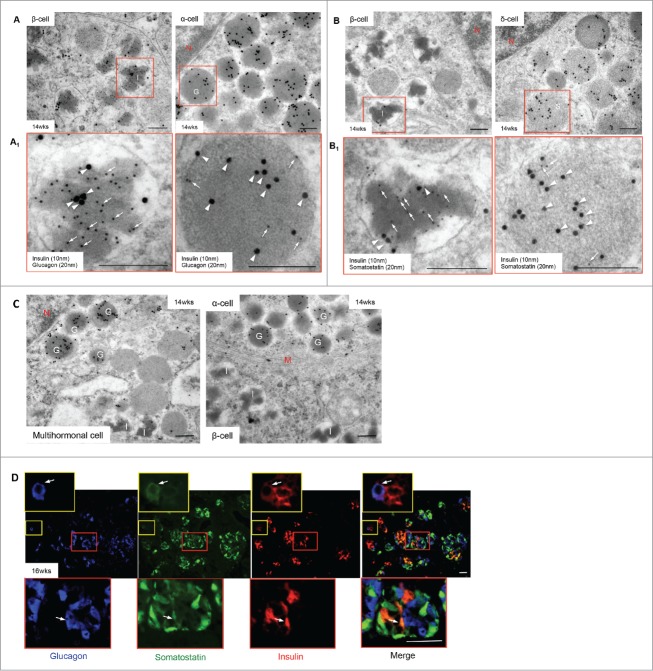
Figure 5.
Polyhormonal granules and multihormonal cells in the developing human fetal pancreas. Transmission electron micrographs of double immunogold staining for insulin and glucagon (A) or insulin and somatostatin (B) with highlighted areas enlarged in (A1) and (B1) in a 14 week human fetal pancreas. A multihormonal (insulin and glucagon) cell (C, left panel) and 2 monohormonal (glucagon above, insulin below) cells (C, right panel) are also shown. Secondary gold antibodies for insulin (10 nm, arrows), glucagon and somatostatin (20 nm, arrowheads). Scale bars = 250 nm. (D) Triple immunofluorescence staining of glucagon (blue), somatostatin (green) and insulin (red), indicating that specific cells are positive for all 3 hormones (arrows) in the human fetal pancreas at 16 weeks fetal age. Highlighted areas are enlarged in the top and bottom insets, scale bar = 50 μm. G = glucagon granules, I = insulin granules, M = plasma membranes, N = nucleus, S = somatostatin. Representative images from 8–10 pancreata are shown.
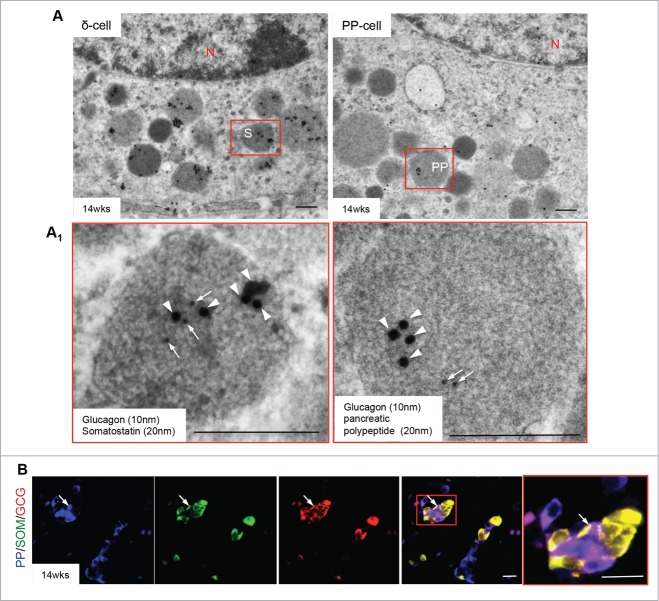
Figure 6.
Glucagon signals present in somatostatin and pancreatic polypeptide granules. Double immunogold staining reveals glucagon (10 nm, arrows) and somatostatin (20 nm, arrowheads) (A, left panel) or pancreatic polypeptide (20 nm, arrowheads) (A, right panel) positivity in a single endocrine cell granule in a 14 week human fetal pancreas. Highlighted areas are enlarged in (A1). Scale bar = 250 nm. (B) Triple immunofluorescence staining of pancreatic polypeptide (blue), somatostatin (green) and glucagon (red) reveals specific cells positive for multiple hormones (arrows) in the 14 weeks fetal pancreas. Highlighted area is enlarged in far right panel, scale bar = 50 μm. N = nucleus, PP = pancreatic polypeptide granules, S = somatostatin granules. Representative images from 8–10 pancreata are shown.
Polyhormonal granules and multihormonal cells as well as intermediate endocrine/exocrine cells in the developing human fetal pancreas
Polyhormonal granules and multihormonal endocrine cells were frequently observed in 14–16 week human fetal pancreata determined by double immunogold labeled TEM and triple immunofluorescent staining (Figs. 5 and 6). Double immunogold labeling revealed that insulin granules often contained either glucagon (Fig. 5A/A1, left panels) or somatostatin (Fig. 5B/B1, left panels), while glucagon and somatostatin granules contained insulin signals (Fig. 5A/A1, right panels; 5B/B1, right panels). Multihormonal cells containing both insulin and glucagon granules were also observed in endocrine cells of the human fetal pancreas (Fig. 5C, left panel) while triple immunofluorescence staining identified numerous cells with weak insulin expression that also had glucagon and somatostatin positivity (Fig. 5D). In addition, somatostatin or pancreatic polypeptide positive granules contained immunoreactivity for glucagon (Fig. 6A/A1), which was confirmed by triple immunofluorescence (Fig. 6B).
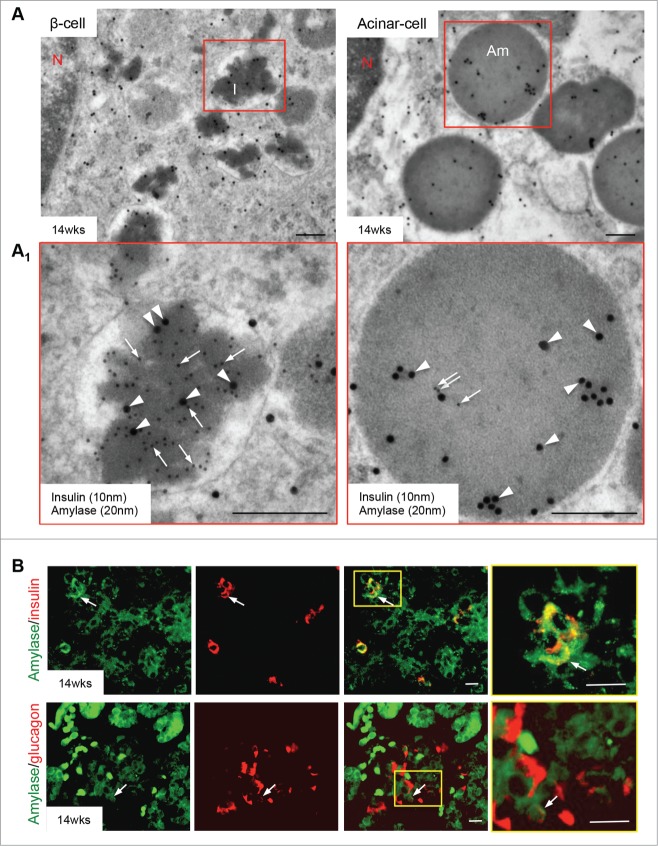
Finally, we identified intermediate endocrine-exocrine cells in a 14 week fetal pancreas using TEM double immunogold and double immunostaining approaches: interestingly, some insulin granules showed positive labeling for amylase, while immature zymogen granules also contained insulin-positive signals (Fig. 7A/A1). It was more common to observe amylase-positivity in insulin granules rather than insulin-positivity in zymogen granules (Fig. 7A/A1). These latter granules were rare, and as the pancreas aged, all cells with these characteristics were less prevalent. Further evidence for this phenomena was observed with double immunostaining, in which some insulin and glucagon positive cells colocalized with amylase (Fig. 7B).
Figure 7.
Co-expression of amylase with insulin in the human fetal pancreas. (A) Double immunogold staining of insulin (10 nm, arrows) and amylase (20 nm, arrowheads) in the human fetal pancreas at 14 weeks fetal age, with highlighted areas enlarged in A1. Scale bars = 250 nm. (B) Double immunofluorescence stain of amylase (green) with either insulin (red) [upper row] or glucagon [lower row] in the human fetal pancreas at 14 weeks of fetal age. Highlighted areas are enlarged in the far right panels: they suggest co-expression of amylase with insulin and glucagon (arrows). Scale bar = 50 μm. Am = amylase, I = insulin, N = nucleus. Representative images from 8–10 pancreata are shown.
Discussion
In the present study, we observed that, during the early stages of its development, the human pancreas consisted primarily of a ductal tree, ECM proteins/fibers and a few cell clusters bordering the ducts. As development continued, the cell clusters became separate from the ductal tree and intercalated with the ECM, eventually forming the endocrine islets and exocrine acini of a more mature pancreas by 20 weeks fetal age. Glycogen was strongly expressed in 8 and 15 week pancreatic ductal cells and lightly in newly formed endocrine cell clusters, but this expression shifted to acinar cell clusters by 20 weeks. Insulin granule maturation was associated with the appearance of multi-lobed geometric structures by 14 weeks. The developing human pancreas also initially contained cells expressing multiple hormones; the majority of these cells became monohormonal by mid-gestation. Of particular interest was the finding of insulin or glucagon and amylase expressed within the same cells, indicating that the intermediate endocrine-exocrine cells, described in non-human species, are also present in the human fetal pancreas.17-19
Our ultrastructural analysis of human fetal insulin granules demonstrated spherical monolobed structures at 8 weeks fetal age which turned into multi-lobed geometric crystal granules by 20 weeks. Mature insulin granule structures differ significantly between rodents and humans.20 The unique crystalline structure of mature human insulin granules has implications for human embryonic stem cell (hESC)-derived insulin-producing cells: their insulin granules more closely resemble mature mouse insulin granules21 or the immature human fetal insulin forms, suggesting that problems associated with hESC-derived β-cell development may involve unsatisfactory insulin granule maturation.
In the present study, cells containing polyhormonal granules or multiple monohormonal granules were often observed in the 8–16 week human fetal pancreas. In each case where there were polyhormonal granules, the granule had a primary hormone that had very strong positive staining, with a lower expression level of a secondary hormone. These data indicate that setting up cellular machinery is a primary goal for early pancreatic cells, rather than lineage determination. Our findings are supported by 2 previous immunogold studies of the human fetal pancreas, which demonstrated that individual cells had polyhormonal granules containing various combinations of insulin, glucagon, somatostatin and pancreatic polypeptide.12,14 The fact that the polyhormonal cells are less frequent at 20 weeks indicates an ongoing maturation process within the endocrine pancreas.
The current study supports the idea of a common progenitor cell for both the endocrine and exocrine pancreas by demonstrating, for the first time in the human, co-expression of endocrine hormones (insulin and glucagon) with an exocrine enzyme (amylase). Intermediate cells that express insulin and amylase in separate granules have been identified in the pancreas of adult rats that have undergone pancreatic duct ligation,17 and in the normal pancreas of the adult rat, guinea-pig, monkey, goat, chicken and frog, where the cells contain immature zymogen granules along with insulin, glucagon or somatostatin hormone-containing granules.18,19 These intermediate cells are described as changing from one differentiated cell state to another in response to demands of the organism, but this process is not well understood.
The present ultrastructural and immunohistochemical analysis of human fetal pancreatic development from early to midgestation provides a better understanding of the processes and intracellular structures involved. These details may help to improve differentiation protocols, including those for hESCs that are essential for generating an unlimited source of β-cells for use in cell-based therapies for the treatment of diabetes.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Mr. David Mallot, in the Department of Pathology at the Western University Hospital, for his assistance with tissue process and sections preparation for the electron microscopy.
Funding
This work is supported by the Canadian Diabetes Association (Grant number: OG-3-11-3318-RW).
References
- 1. Piper K, Brickwood S, Turnpenny LW, Cameron IT, Ball SG, Wilson DI, Hanley NA. Beta cell differentiation during early human pancreas development. J Endocrinol 2004; 181:11-23; PMID:15072563; http://dx.doi.org/ 10.1677/joe.0.1810011 [DOI] [PubMed] [Google Scholar]
- 2. Scharfmann R. Control of early development of the pancreas in rodents and humans: implications of signals from the mesenchyme. Diabetologia 2000; 43:1083-92; PMID:11043853; http://dx.doi.org/ 10.1007/s001250051498 [DOI] [PubMed] [Google Scholar]
- 3. McCracken KW, Wells JM. Molecular pathways controlling pancreas induction. Semin Cell Dev Biol 2012; 23:656-62; PMID:22743233; http://dx.doi.org/ 10.1016/j.semcdb.2012.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lyttle BM, Li J, Krishnamurthy M, Fellows F, Wheeler MB, Goodyer CG, Wang R. Transcription factor expression in the developing human fetal endocrine pancreas. Diabetologia 2008; 51:1169-80; PMID:18491072; http://dx.doi.org/ 10.1007/s00125-008-1006-z [DOI] [PubMed] [Google Scholar]
- 5. Skandalakis LJ, Rowe JS, Jr, Gray SW, Skandalakis JE. Surgical embryology and anatomy of the pancreas. Surg Clin N Am 1993; 73:661-97; PMID:8378816 [DOI] [PubMed] [Google Scholar]
- 6. Like AA, Orci L. Embryogenesis of the human pancreatic islets: a light and electron microscopic study. Diabetes 1972; 21:511-34; PMID:4559917 [DOI] [PubMed] [Google Scholar]
- 7. Jeon J, Correa-Medina M, Ricordi C, Edlund H, Diez JA. Endocrine cell clustering during human pancreas development. J Histochem Cytochem 2009; 57:811-24; PMID:19365093; http://dx.doi.org/ 10.1369/jhc.2009.953307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meier JJ, Kohler CU, Alkhatib B, Sergi C, Junker T, Klein HH, Schmidt WE, Fritsch H. Beta-cell development and turnover during prenatal life in humans. Eur J Endocrinol 2010; 162:559-68; PMID:20022941; http://dx.doi.org/ 10.1530/EJE-09-1053 [DOI] [PubMed] [Google Scholar]
- 9. Bouwens L, Pipeleers DG. Extra-insular β-cells associated with ductules are frequent in adult human pancreas. Diabetologia 1998; 41:629-33; PMID:9662042; http://dx.doi.org/ 10.1007/s001250050960 [DOI] [PubMed] [Google Scholar]
- 10. Inada A, Nienaber C, Katsuta H, Fujitani Y, Levine J, Morita R, Sharma A, Bonner-Weir S. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci U S A 2008; 105:19915-9; PMID:19052237; http://dx.doi.org/ 10.1073/pnas.0805803105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Laitio M, Lev R, Orlic D. The developing human fetal pancreas: an ultrastructural and histochemical study with special reference to exocrine cells. J Anat 1974; 117:619-34; PMID:4418855 [PMC free article] [PubMed] [Google Scholar]
- 12. Bocian-Sobkowska J, Zabel M, Wozniak W, Surdyk-Zasada J. Polyhormonal aspect of the endocrine cells of the human fetal pancreas. Histochem Cell Biol 1999; 112:147-53; PMID:10460468; http://dx.doi.org/ 10.1007/s004180050401 [DOI] [PubMed] [Google Scholar]
- 13. Riedel MJ, Asadi A, Wang R, Ao Z, Warnock GL, Kieffer TJ. Immunohistochemical characterisation of cells co-producing insulin and glucagon in the developing human pancreas. Diabetologia 2012; 55:372-81; PMID:22038519; http://dx.doi.org/ 10.1007/s00125-011-2344-9 [DOI] [PubMed] [Google Scholar]
- 14. Lukinius A, Ericsson JL, Grimelius L, Korsgren O. Ultrastructural studies of the ontogeny of fetal human and porcine endocrine pancreas, with special reference to colocalization of the four major islet hormones. Dev Biol 1992; 153:376-85; PMID:1356860; http://dx.doi.org/ 10.1016/0012-1606(92)90122-W [DOI] [PubMed] [Google Scholar]
- 15. De Krijger RR, Aanstoot HJ, Kranenburg G, Reinhard M, Visser WJ, Bruining GJ. The midgestational human fetal pancreas contains cells coexpressing islet hormones. Dev Biol 1992; 153:368-75; PMID:1356859; http://dx.doi.org/ 10.1016/0012-1606(92)90121-V [DOI] [PubMed] [Google Scholar]
- 16. Walesky C, Gunewardena S, Terwilliger EF, Edwards G, Borude P, Apte U. Hepatocyte-specific deletion of hepatocyte nuclear factor-4alpha in adult mice results in increased hepatocyte proliferation. Am J Physiol Gastrointest Liver Physiol 2013; 304:G26-37; PMID:23104559; http://dx.doi.org/ 10.1152/ajpgi.00064.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bertelli E, Bendayan M. Intermediate endocrine-acinar pancreatic cells in duct ligation conditions. Am J Physiol 1997; 273:C1641-9; PMID:9374650 [DOI] [PubMed] [Google Scholar]
- 18. Melmed RN, Benitez CJ, Holt SJ. Intermediate cells of the pancreas. I. Ultrastructural characterization. J Cell Sci 1972; 11:449-75; PMID:4627700 [DOI] [PubMed] [Google Scholar]
- 19. Leduc EH, Jones EE. Acinar-islet cell transformation in mouse pancreas. J Ultrastruct Res 1968; 24:165-9; PMID:4879094; http://dx.doi.org/ 10.1016/S0022-5320(68)80026-7 [DOI] [PubMed] [Google Scholar]
- 20. Xue Y, Zhao W, Du W, Zhang X, Ji G, Ying W, Xu T. Ultra-structural study of insulin granules in pancreatic beta-cells of dbdb mouse by scanning transmission electron microscopy tomography. Protein Cell 2012; 3:521-5; PMID:22773341; http://dx.doi.org/ 10.1007/s13238-012-2937-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rezania A, Bruin JE, Riedel MJ, Mojibian M, Asadi A, Xu J, Gauvin R, Narayan K, Karanu F, O'Neil JJ, et al. . Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes 2012; 61:2016-29; PMID:22740171; http://dx.doi.org/ 10.2337/db11-1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.