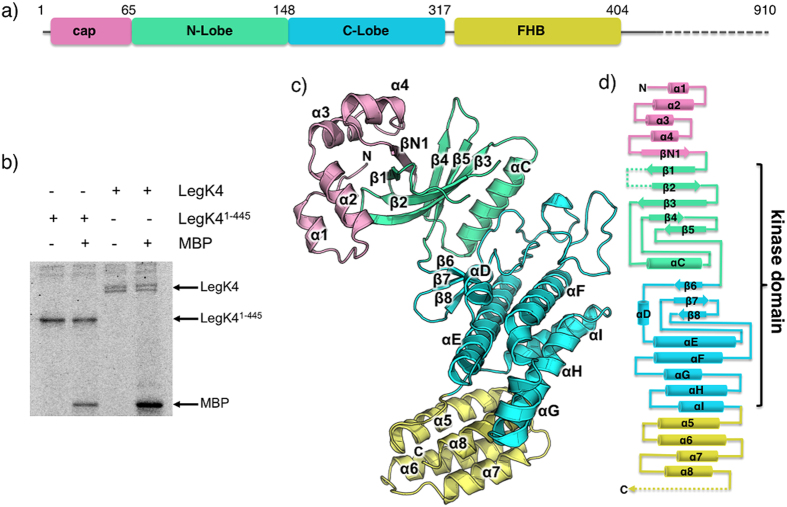
Figure 1. LegK41–445 crystal structure.
(a) Schematic representation of LegK41–445 protein sequence and the structurally assigned domains of LegK41–445. The “cap” domain (residues 1-65) is represented in pink, the kinase domain is separated in two lobes: the N-lobe (residues 66-148, green) and the C-lobe (residues 149-317, light blue) and the four-helix bundle (FHB) domain (residues 318-406) is coloured in yellow. (b) Purified LegK proteins were subjected to in vitro auto- and myelin basic protein (MBP) phosphorylation assays in the presence of [γ-32P]ATP. Phosphoproteins were separated by SDS-PAGE and visualised by autoradiography. (c) Ribbon representation of the crystal structure of LegK41–445 with domains coloured as in (a). (d) Topological diagram of LegK41–445, using the same colour code as in panels (a,c). The missing unstructured regions are represented as dashed lines.