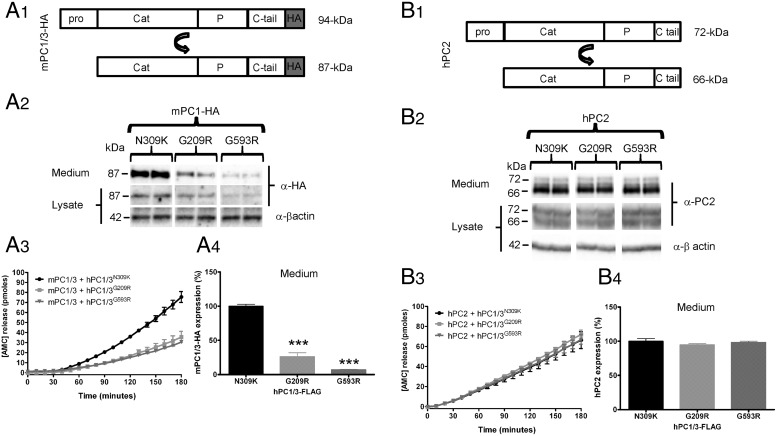
Figure 4.
PC1/3 ER-retained mutants cause a dominant-negative effect on wild-type enzyme. A1, A HA-tagged wild-type mPC1/3 construct (mPC1/3-HA) was used to study the effect of PC1/3 ER-retained mutants in Neuro2A cells; the well-secreted PC1/3 N309K mutant was used as a control. Transfections were performed using mPC1/3-HA in the presence of PC1/3 mutants at a 4:1 stoichiometry (wild-type: mutant). A2, mPC1/3-HA expression was analyzed using the 16B12 anti-HA antibody in media and cell lysate samples. Only the 87-kDa PC1/3 form was detected by Western blotting. A3, mPC1/3 activity was measured using the standard fluorogenic enzymatic assay. A4, Wild-type PC1/3-HA expression, from media samples depicted in A3, is significantly reduced when PC1/3 ER-retained mutants are coexpressed. B1, Nontagged hPC2 was used to test the specificity of the dominant-negative effect. B2, The hPC2 72-kDa zymogen form and the 66-kDa mature form were detected by Western blotting using propeptide and amino-terminal PC2 antisera. B3, hPC2 activity was measured using the standard fluorogenic enzymatic assay. B4, Analysis of hPC2 expression, from media samples analyzed in B3, does not show significant differences when PC1/3 ER-retained mutants are coexpressed. Expression level data were analyzed using a one-way ANOVA followed by a post hoc Bonferroni's test (***, P < .001; **, P < .01; *, P < .05); n = 3.