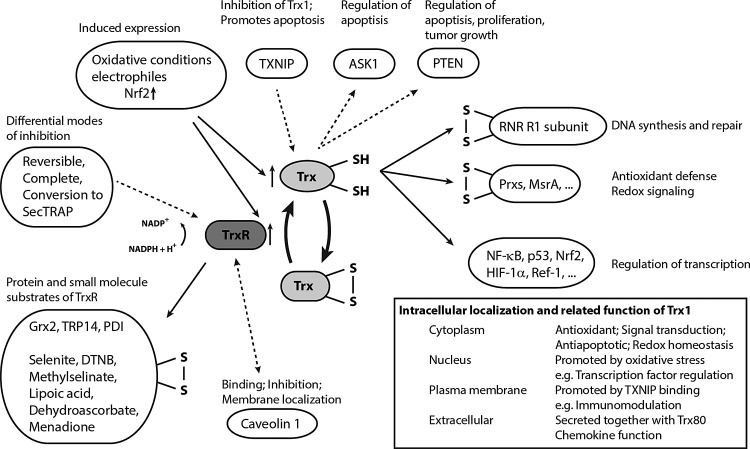
FIG. 4.
Substrates and principle functions of the thioredoxin system. This scheme summarizes in greater detail the diverse functions of the Trx system, as well as the possible direct reactions involving TrxR. Dotted lines indicate direct protein–protein binding or modification, whereas solid lines denote redox activity and thiol–disulfide exchange reactions. Expression of both Trx and TrxR is induced via Nrf2 under various stress conditions (Fig. 2). Trx1 is predominantly located in the cytosol, where it provides ribonucleotide reductase (RNR) with electrons and supports the activity of Prxs (255) and Msrs (175). Trx1 can also translocate to the nucleus, where it regulates gene expression by modulating transactivation of various transcription factors, including NFκB, HIF, p53, Nrf2, AP-1, and the glucocorticoid receptor (8, 93, 99, 103, 112, 125, 126, 298). Furthermore, reduced Trx1 directly binds PTEN, a major tumor suppressor that prevents survival signaling by deactivating the PI3K/Akt pathway. Trx1 binding inhibits the phosphatase activity of PTEN and thus promotes cell proliferation and tumor growth while also inhibiting apoptosis (217). Trx1 is also an important regulator of apoptosis signal-regulating kinase 1 (ASK1). In its reduced form, Trx1 binds and thus inhibits ASK1. However, high levels of reactive oxygen species (ROS) promote oxidation of Trx1 and thus ASK1 release, leading to subsequent apoptosis (264). ASK1 release may also be promoted by the Trx1-interacting protein (TXNIP), an endogenous inhibitor of Trx1 that binds to reduced Trx1 and thus competes with ASK1 (327). Interestingly, TXNIP binding also mediates Trx1 translocation to the plasma membrane, which is proposed to enable inflammation in endothelial cells by promoting cell survival and vascular endothelial growth factor signaling during oxidative stress (319). In addition, Trx1 together with a truncated variant (Trx80) can be found in the extracellular environment where it exhibits an oxidoreductase-independent chemokine-like activity (232, 243). TrxR1 also catalyzes the reduction of various additional thiol-proteins and low-molecular-weight compounds and is a prime target for many electrophilic drugs (11). This figure is a modified version of a figure in a review from Lu and Holmgren (197).