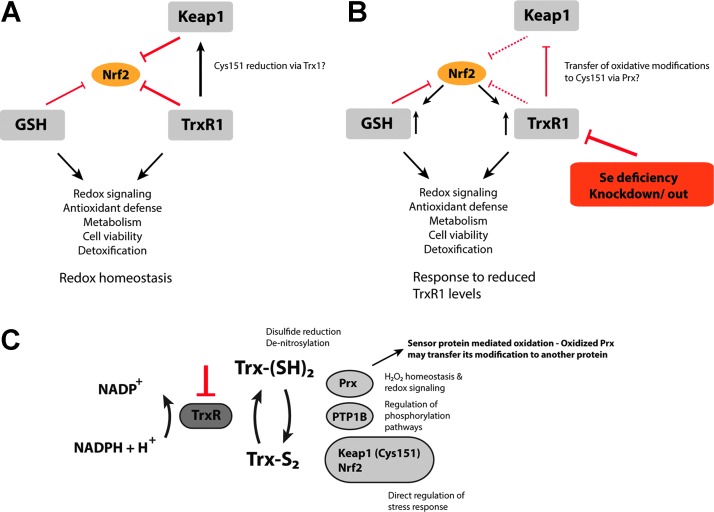
FIG. 6.
TrxR1 as an essential negative regulator of Nrf2. (A) Normal, unstressed cells with the Trx- and GSH systems expressed at a basal level maintain redox homeostasis. Both systems act, together with Keap1, as negative regulators of Nrf2 transactivation counteracting oxidative and electrophilic insults. Furthermore, TrxR1 might directly prevent Keap1 inhibition by reducing the critical cysteine 151 via Trx1. (B) A reduction in the catalytic capacity of TrxR1 either by Se deficiency or due to knockdown or knockout leads to activation of Nrf2. This, in turn, promotes the expression of various enzymes of the Trx and GSH systems, boosting the antioxidant and detoxification capacity of the cell. (C) Loss of TrxR1 activity leads to direct interplay with Nrf2 signaling through several different mechanisms. The mechanisms of TrxR1 targeting leading to Nrf2 activation likely involve combinations of a reduced antioxidant capacity, changes in redox signaling-dependent pathways (particularly those mediated by Trx1), and direct regulatory effects on Keap1 and Nrf2. The lack of TrxR1 prevents Trx1 from its reductive functions, which leads to oxidation of Cys151 in Keap1, either directly or potentially via a transfer of oxidative equivalents from Prx (257). This latter mechanism would serve as an “oxidative switch” in the regulation of Keap1, as not only reduction is diminished but also oxidation is actively promoted. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars