Abstract
Although the performance of simple cognitive tasks can be enhanced if an incentive is provided, the mechanisms enabling such motivational control are not known. The present study sought to uncover how mechanisms of attention and readiness are altered by reward-associated incentive stimuli. We measured EEG/ERP activity as human adults viewed a high- or low-incentive cue, experienced a short preparation interval, and then performed a simple visual search task to gain the predicted reward. Search performance was faster with high versus low incentives, and this was accompanied by distinct incentive-related EEG/ERP patterns at each phase of the task (incentive, preparation, and search). First, and most surprisingly, attention to high but not low incentive cues was actively suppressed, as indexed by a PD component in response to the incentive display. During the subsequent preparation interval, neural oscillations in the alpha frequency range were reduced following high-incentive cues, indicating heightened visual readiness. Finally, attentional orienting to the target in the search array was deployed with relatively little effort on high-incentive trials, as indexed by a reduced N2pc component. These results reveal the chain of events by which the brain’s executive control mechanisms respond to incentives by altering the operation of multiple processing systems to produce optimal performance.
Keywords: Incentive, Motivation, Cognitive Enhancement, Attention, Event-Related Potential, Neural Oscillation
INTRODUCTION
In many situations, cues in the environment reliably signal an upcoming opportunity to gain a sought-after reward. For example, a friendly smile typically signals an opportunity to gain social approval. As a consequence of learning, cues can eventually serve to enhance motivation for the associated reward (Berridge, 2012) and thereby facilitate the behavior needed to acquire it. Although it has been shown that incentives can improve performance in a wide range of situations such as motor initiation and execution (Mir et al., 2011) and visual cognition (Small et al., 2005), the mechanisms that lead from the detection of incentive stimuli to enhanced performance are far from fully specified. A particularly poorly understood piece of this puzzle is how incentives modify internal cognitive and attentional processes.
Previous studies have shown that incentives can increase the speed of accurate responses on perceptual detection or recognition tasks by enhancing motor preparation (Mir et al., 2011). However, in many situations where response speed is not instrumental for reward acquisition, motivation is more likely to enhance cognitive rather than motor processes. Yet, surprisingly, whether and how incentives improve cognitive processing is largely unknown. Previous studies demonstrating incentive-induced performance enhancements in simple visual search tasks confounded motor and cognitive preparation opportunities by giving participants cues that allowed them to prepare both the required motor and attentional responses before the task stimuli were presented (Engelmann & Pessoa, 2007; Small et al., 2005). Thus incentive effects reported in these studies could have resulted solely from motor preparation (Mir et al., 2011), leaving open the question of whether incentives can improve cognitive processing. Other studies have shown that visual search is faster and more accurate when the search target itself is associated with reward (Hickey, Chelazzi, & Theeuwes, 2010; Kiss, Driver, & Eimer, 2009). In these studies, a single stimulus served as both incentive cue and target, and a speeded response to it was required, precluding any opportunity for cognitive preparation. Thus, these studies provide little insight into whether incentives can change the efficiency of the cognitive processing of subsequent presented stimuli.
To investigate this issue, we devised a task in which one of two possible cues signaled the availability of a large or small reward that could only be obtained by fast, accurate response to an imminent visual display. Importantly, the cue did not predict the specific motor response required but did offer an opportunity to engage in cognitive preparation. EEG-based measures were used to determine which specific aspects of brain activity were influenced by incentive stimuli during various phases of the task.
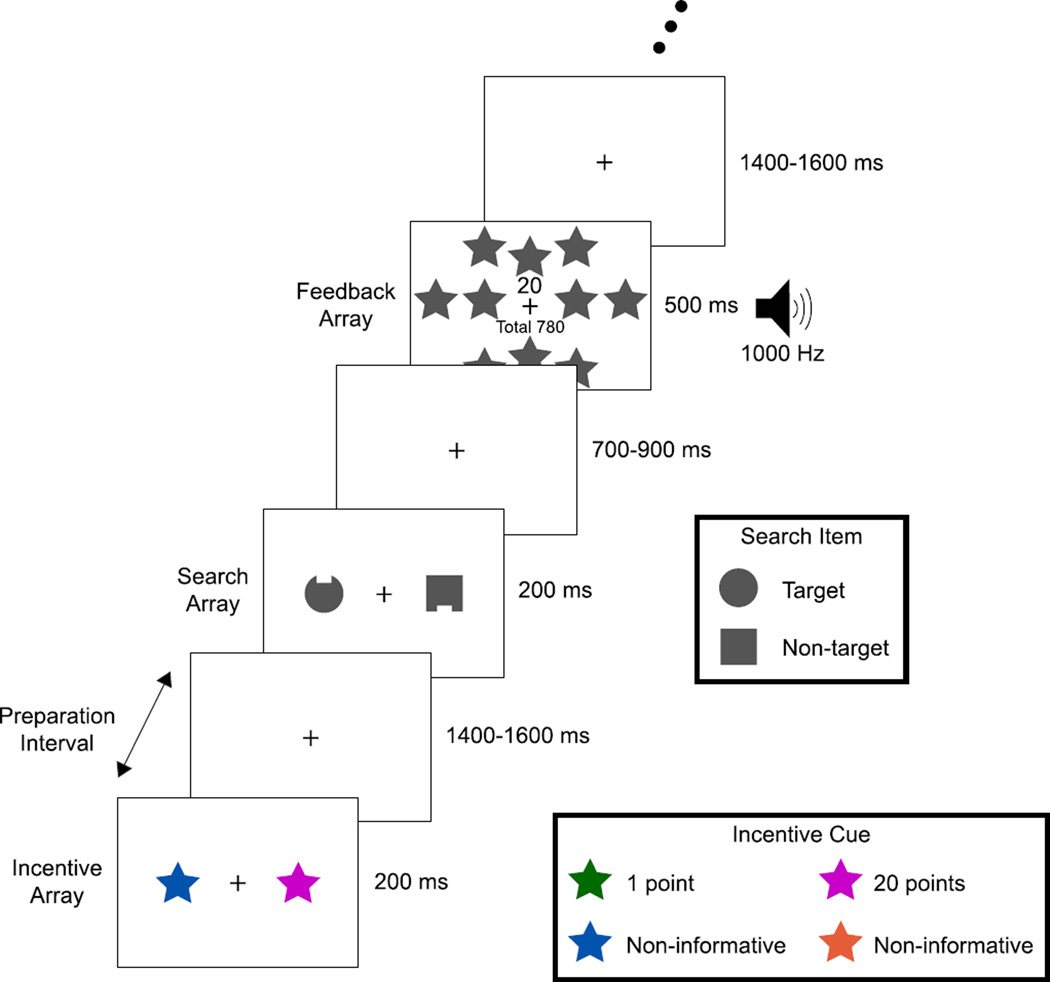
Each trial of this task had three parts: 1) an incentive array that signaled the possibility of winning a small or large reward on that trial; 2) a short (~1500 ms) preparation interval; and 3) a simple two-object visual search array (see Fig. 1). Participants reported the location (top or bottom) of a small notch on a target object in the search array as quickly and accurately as possible to win the reward indicated by the preceding incentive array. The target object was always a circle and the non-target was always a square (reversed for half of participants). The unpredictability of the target’s location and notch position discouraged anticipatory spatial orienting and specific motor preparation. We examined event-related potentials (ERPs) elicited by the incentive array, time-frequency representations (TFRs) of the EEG during the subsequent preparation interval, and ERP and behavioral responses to the search array. Thus, we were able to investigate incentive-related processes across multiple distinct phases of the task, reflecting the phases that are present in real-world situations in which incentives are provided in advance of a task.
Fig 1.
Participants viewed an incentive array composed of an incentive star of one color and a non-informative star of another color (counterbalanced across participants). After ~1500 ms with only a fixation cross present, a visual search array composed of a circle and square appeared for 200 ms; both shapes were grey and contained a notch at the top or bottom. One shape served as target, the other as non-target (counterbalanced across participants). The task was to report as quickly and accurately as possible (by pressing the corresponding game pad button) whether the target’s notch was on the top or bottom. Points awarded for that trial and a running total were indicated on a feedback display seen for 500 ms. Points awarded (later exchanged for cash) were contingent on correct responses; crucially, the amount earned on each trial depended reliably on the color of the incentive star viewed on that trial. The location of the incentive star, target, and notch were randomized and unrelated to each other. Bilateral arrays were used to facilitate extraction of the N2pc and PD ERP components.
By their very nature, incentive stimuli have strong reward associations. Recent studies show that when stimuli are associated with rewarding outcomes, they are recognized more quickly (O’Brien & Raymond, 2012), are more likely to capture attention (Anderson, Laurent, & Yantis, 2011), and result in a durable high-level visual representation (Raymond & O’Brien, 2009). Thus, one possibility for the current experiment was that we would observe greater allocation of attention to high-incentive cues than to low-incentive cues. Even though a strong focus of attention may be beneficial for many types of reward-related stimuli, it could be counterproductive for cues that signal the potential reward value of an upcoming task. For example, if a video game player becomes strongly focused on a cue that indicates the availability of a large reward (e.g., a flashing “4X!” sign indicating each target is worth quadruple points), this focused attention would interfere with performing the task needed to obtain the reward (e.g., hitting the incoming targets). Thus, it may be necessary to suppress the allocation of attention to incentive cues so that cognitive performance can be optimized for the upcoming task. We tested these two possibilities by recording the ERPs elicited by the incentive cues and examining the N2pc and PD ERP components, which reflect deployment of covert attention (Luck, 2012; Luck & Hillyard, 1994a, 1994b) and active suppression of attention (Hickey, Di Lollo, & McDonald, 2009; Sawaki, Geng, & Luck, 2012), respectively. The N2pc component is observed as a more negative voltage at contralateral scalp sites than at ipsilateral scalp sites relative to the position of an attended stimulus. In contrast, the PD component is observed as a more positive voltage at contralateral scalp sites than at ipsilateral scalp sites relative to the position of a suppressed stimulus. Both components typically begin 150–250 ms after the onset of the stimulus presentation, depending on stimulus properties and task demands. The N2pc component appears to be generated in intermediate and high levels of the ventral visual processing pathway (i.e., V4 and the lateral occipital complex; Hopf et al., 2006; Hopf, Boelmans, Schoenfeld, Luck, & Heinze, 2004). Neural sources of the PD component are currently not determined. However, the PD and N2pc component have similar scalp distributions with opposite polarities and complementary roles in attention, and thus it is plausible that these components are associated with opposing attentional processes within the same neural sources. In the present study, if incentive cues trigger the allocation of attention, then they should elicit an N2pc component. If, in contrast, people actively suppress incentive cues so that they can optimize their cognitive performance on the upcoming task, then they should elicit a PD component.
To determine how incentives lead to changes in neural processing that might enhance performance, we examined EEG oscillations during the preparation period between the incentive cue and the visual search target. In particular, decrements in alpha power are thought to reflect greater readiness to process external stimuli (Mazaheri et al., 2014; Mazaheri, Nieuwenhuis, van Dijk, & Jensen, 2009). We therefore predicted that alpha power would be reduced following a cue indicating the availability of a large (versus small) reward.
Although incentives might facilitate attentional processes, as indexed by the target-elicited N2pc component, it is not clear how this would be achieved. One possibility is that the N2pc might be larger on high-reward trials, just as it is larger when a single stimulus is both a high-incentive cue and a rewarding target (Kiss et al., 2009). The other possibility is that the N2pc might actually be smaller on high-reward trials, just as it is smaller for stimuli that can be more easily discriminated (Anderson, Ester, Klee, Vogel, & Awh, 2014; Burra & Kerzel, 2014; Eimer, 1996; Luck & Hillyard, 1994b). This would be analogous to the smaller BOLD responses that are observed for primed stimuli (Buckner et al., 1998; Henson, 2003).
METHODS
Participants
Thirteen neurologically normal volunteers between 18 and 30 years old participated in exchange for money ($10/hour plus bonus payment; see below). All had normal or corrected-to-normal vision. Informed consent was obtained, and the protocol was approved by the UC-Davis Institutional Review Board.
Stimuli and Procedure
The stimuli were presented on a video monitor with a black background at a distance of 70 cm. The sequence of events on a typical trial is illustrated in Fig. 1. At the beginning of the experiment, two of four colors (orange: u’ = .28, v’ = .53, green: u’ = .15, v’ = .55, blue: u’ = .17, v’ = .33, or pink: u’ = .28, v’ = .28; luminance = 20 cd/m2 for all colors) were designated as the low- and high-incentive colors, respectively, and the other two were designated as non-informative colors. In addition, either a circle (1.6° diameter) or a square (1.3° width) was designated as the target. The incentive color assignment and target shape were counterbalanced across participants. Note that each array contained two items, one relevant and one irrelevant, so that we could examine lateralized attention-related ERP responses without low-level physical stimulus confounds.
Each trial sequence consisted of an incentive array, a preparation interval, a search array, and finally a feedback array. A grey fixation cross (0.3°×0.3°) was continuously visible at the center of the display. Following a blank inter-trial interval (1400–1600 ms, rectangular distribution), the incentive array was presented for 200 ms. Each incentive array consisted of two star shapes (2°×2°, centered 2.5° to the left and right of fixation); one was drawn in either the low- or the high-incentive color, and the other was drawn using one of the two non-informative colors. The side of the array containing the incentive color and the non-informative color varied randomly across trials so that we could measure the N2pc and PD components, which are lateralized with respect to the location of the relevant item. The presence of a low- or high-incentive cue predicted that a correct response in the following search array would be rewarded with 1 or 20 points, respectively. Participants received $1 per 1000 points as a bonus payment (maximum $8, average $7.2).
After a preparation interval (1400–1600 ms, rectangular distribution), a search array was presented for 200 ms. Each search array consisted of a gray circle and a gray square, centered 2.5° to the left and right of fixation. Both items had a notch (0.2° tall×0.6° wide) on the top or the bottom, and the location of the notch on each item varied randomly and independently across trials. Participants were instructed to report the location of the target’s notch (top or bottom) by pressing one of two spatially corresponding buttons on a game pad with the index and middle fingers of the right hand as quickly and accurately as possible. We used this highly simplified visual search task because it facilitates the measurement of attention-related ERP components. The interval between search array offset and the following feedback array was relatively short (700–900 ms), and participants needed to make a response during this interval. Therefore, a relatively fast response was required to gain reward.
The feedback array and tone were presented for 500 ms to inform participants of the outcome of the search task for that trial. If the search response was correct, a “1” or “20” was shown on the display to indicate the points awarded, and a 500 or 1000 Hz tone was sounded. To make the distinction between low- and high-incentive trials especially salient, the high-incentive feedback display also contained a cluster of ten gray stars. Incorrect or missing responses led to no points; feedback in these situations consisted of a 200 Hz tone and a display of “Wrong response” or “No response”, as appropriate. On all trials a running total of points (e.g., “Total 780”) was also presented. All sounds had an intensity of approximately 60 dB and were easily discriminated.
The experimental block comprised 32 practice trials followed by 24 blocks of 32 trials during which EEG was recorded. Participants were explicitly instructed that the location of the incentive cue did not predict the location of the target item or the location of the target notch. They were required to maintain central fixation throughout the trial, verified with electrooculogram (EOG) recordings.
Prior to the main experimental session, each participant was explicitly instructed about the associations between the colors used in the incentive array and the points that would be awarded for correct responses in the search task. After this instruction, they completed a short learning session to reinforce the associations: Following a blank inter-trial interval (1400–1600 ms, rectangular distribution), a number “1” or “20” was randomly presented at the center of display for 200 ms. After a delay (1400–1600 ms, rectangular distribution), two star shapes were presented at the left and right of fixation for 200 ms, just as with the incentive array in the main experiment. One was colored in either the low- or the high-incentive color, corresponding to the number presented in the previous array (i.e., “1” was the low-incentive color, “20” was the high-incentive color), and the other was colored using one of the non-informative colors. The location of the low- or high-incentive color and the non-informative color varied randomly across trials. Participants were instructed to report the location of the color associated with the number in the previous array by pressing one of two spatially corresponding buttons on a game pad with the right hand. After a delay (1100–1300 ms, rectangular distribution), like the main experiment, a feedback array and tone were presented for 500 ms to inform participants of the outcome on the previous association task (“1”, “20”, “Wrong response” or “No response”). The training period comprised 64 trials, and all participants showed perfect performance by the end of this period.
Recording and Analysis
The EEG was recorded using active Ag/AgCl electrodes (BioSemi ActiveTwo) from the left and right mastoids and 32 scalp sites (Fp1, Fp2, F7, F3, Fz, F4, F8, T7, C3, Cz, C4, T8, P9, P7, P5, P3, P1, Pz, P2, P4, P6, P8, P10, PO7, PO3, POz, PO4, PO8, O1, Oz, O2, and Iz), according to the modified 10–20 System (American Electroencephalographic Society, 1994). To detect eye movements and blinks, the EOG was recorded from electrodes placed at the outer canthi of each eye, and above and below the right eye. All signals were recorded in single-ended mode. The EEG and EOG were low-pass filtered with a 5th-order sinc filter (half-power cutoff at 208 Hz) and digitized at 1024 Hz.
All data analyses were performed in Matlab using ERPLAB toolbox (Lopez-Calderon & Luck, 2014), EEGLAB Toolbox (Delorme & Makeig, 2004), and FieldTrip (Oostenveld, Fries, Maris, & Schoffelen, 2011). The EEG signals were referenced offline to the average of the left and right mastoids, and the four EOG signals were referenced into bipolar vertical and horizontal EOG derivations. These signals were bandpass filtered offline using a noncausal Butterworth infinite impulse response filter with half-power cutoffs at 0.05 and 30 Hz and a roll-off of 12 dB/octave and then down-sampled to 256 Hz. The EEG signals were collapsed across stimulus locations and colors to eliminate sensory confounds related to these factors.
Trials were automatically excluded if they contained an incorrect response or if the RT was shorter than 100 ms or longer than 1100 ms. A combination of artifact rejection and artifact correction (via independent component analysis) was used to account for eye blinks. First, to eliminate trials in which the eyes were closed during the period of a stimulus, trials were excluded if the vertical EOG exceeded ±80 µV between 100 ms before and 200 ms after stimulus onset. Then, independent component analysis (ICA) was used to estimate and subtract eyeblink-related voltages in the remaining trials (Jung et al., 2000). Finally, trials were excluded if the EEG exceeded ±100 µV in any channel, if the vertical or horizontal EOG exceeded ±80 µV, or if a step function applied to the horizontal EOG exceeded 15 µV (see chapter 6 in Luck, 2014). To assess whether any systematic horizontal EOG activity was present in the remaining data, we computed averaged horizontal EOG waveforms for left- and right-cue/target trials (see Woodman & Luck, 2003). In all participants, residual EOG activity was less than 3.2 µV, which means that the residual eye movements were less than ±0.1°, with a propagated voltage of less than 0.1 µV at the posterior scalp sites (Lins, Picton, Berg, & Scherg, 1993).
Averaged ERP waveforms were computed with a 600-ms epoch, beginning 100 ms before stimulus onset. The N2pc and PD components were measured from difference waves, in which the waveform from the hemisphere ipsilateral to the stimulus of interest was subtracted from the waveform from the hemisphere contralateral to the stimulus (e.g., the contralateral waveform for the target was the average of the left-hemisphere electrode when the target was in the right visual field and the right-hemisphere electrode when the target was in the left visual field; the ipsilateral waveform for the target was the average of the left-hemisphere electrode when the target was in the left visual field and the right-hemisphere electrode when the target was in the right visual field). Because the overall energy of the stimuli was bilateral, this subtraction eliminates most ERP components, with N2pc and PD remaining in the difference wave (see Luck, 2012 for a detailed justification). The amplitude was measured as the mean voltage during the defined time window (incentive array: N2pc 250–300 ms, PD 325–375 ms; search array: N2pc 260–310 ms), relative to the mean voltage during the 100-ms pre-stimulus baseline period at the PO7 and PO8 electrode sites (where the N2pc and PD are both large). In addition, all statistical results of amplitude analysis were confirmed with nonparametric permutation tests that do not depend on precisely defined measurement windows (Luck, 2014; Sawaki et al., 2012; Sawaki & Luck, 2013). This permutation approach can provide an estimate of the probability that the observed response is due to random variation in the data rather than a consistent physiological response (Ernst, 2004). To perform the permutation test of N2pc and PD components, the side of the stimulus (incentive-cue, target) was randomly recoded and the EEG signals were re-averaged to form a group grand average and difference wave (contralateral minus ipsilateral). Then, negative and positive area values were calculated from the resulting difference waveforms (time-window 100–500 from the stimulus onset). These measurements were performed 1000 times with different randomizations of the coding. If the observed value from the original waveform is greater than 95% of the distribution obtained over these 1000 iterations, we can conclude that the observed contra-ipsilateral difference reflects a consistent physiological response rather than noise in the data. Furthermore, the permutation approach was also used to test whether the observed amplitude difference in target N2pc between low- and high-incentive trials was significantly greater than would be expected by chance. The procedure was the same as that used to test N2pc/PD components (time-window: 100–500 from the target onset, number of iterations: 1000), except as follows. To perform the permutation test, the side of the stimulus was kept but the incentive trial type was randomly recoded (e.g., the left target in low-incentive trials was relabeled as the left target in low- or high-incentive trials, randomly). If the observed value of the N2pc amplitude difference between the low- and high-incentive trials from the original waveform is greater than 95% of the permutation distribution, we can conclude that the observed difference is statistically significant.
Time-frequency representations (TFRs) of power were computed from 2 to 20 Hz by convolving the EEG with a Hanning-tapered five cycle Morlet wavelet. The time-locked ERP activity was subtracted out of each individual trial before TFRs were computed to isolate oscillatory activity not visible in the ERP analysis (Cohen, 2014; Sauseng et al., 2009). To analyze event-related power changes of alpha activity, the power for the individual trials (from −500 ms to 1500 ms relative to the cue array) was measured as % change from the mean voltage during the 500 ms pre-stimulus baseline period and averaged over trial types. To assess incentive-related change in alpha power, we examined the difference in the power of frequencies in the alpha band (10–14 Hz) between conditions across all channels and times. We corrected for multiple comparisons by means of cluster level randomization (Maris & Oostenveld, 2007). Then, a Monte Carlo estimate of the permutation p-value of the cluster was obtained by comparing the cluster-level test statistic to a randomization null distribution assuming no difference between conditions (Jokisch & Jensen, 2007). This distribution is obtained by randomly swapping the conditions in participants 1000 times and calculating the maximum cluster-level test statistic.
RESULTS
Behavior
The mean response time (RT) for producing correct visual search responses was significantly shorter following a high-incentive cue (527 ms) than a low incentive cue (538 ms) [t (12) = −5.2, p < .001]. Moreover, reaction time variability (quantified as the coefficient of variation) was lower on high-incentive trials than on low-incentive trials (7.8 versus 8.1 ms), although this was only marginally significant [F (1,12) = 3.5, p = .087]. Mean accuracy was nearly identical on low- and high-incentive trials (98.0% and 97.8% respectively; t (12) = 0.6). Finding faster responses without a concurrent loss in accuracy shows that performance on a simple visual search can be enhanced by motivation in the absence of cues to prepare a specific motor response. This result also confirms that participants successfully learned to associate different star colors with different reward values, and that they were able to use this information “on the fly” to enhance performance.
Incentive Array ERPs
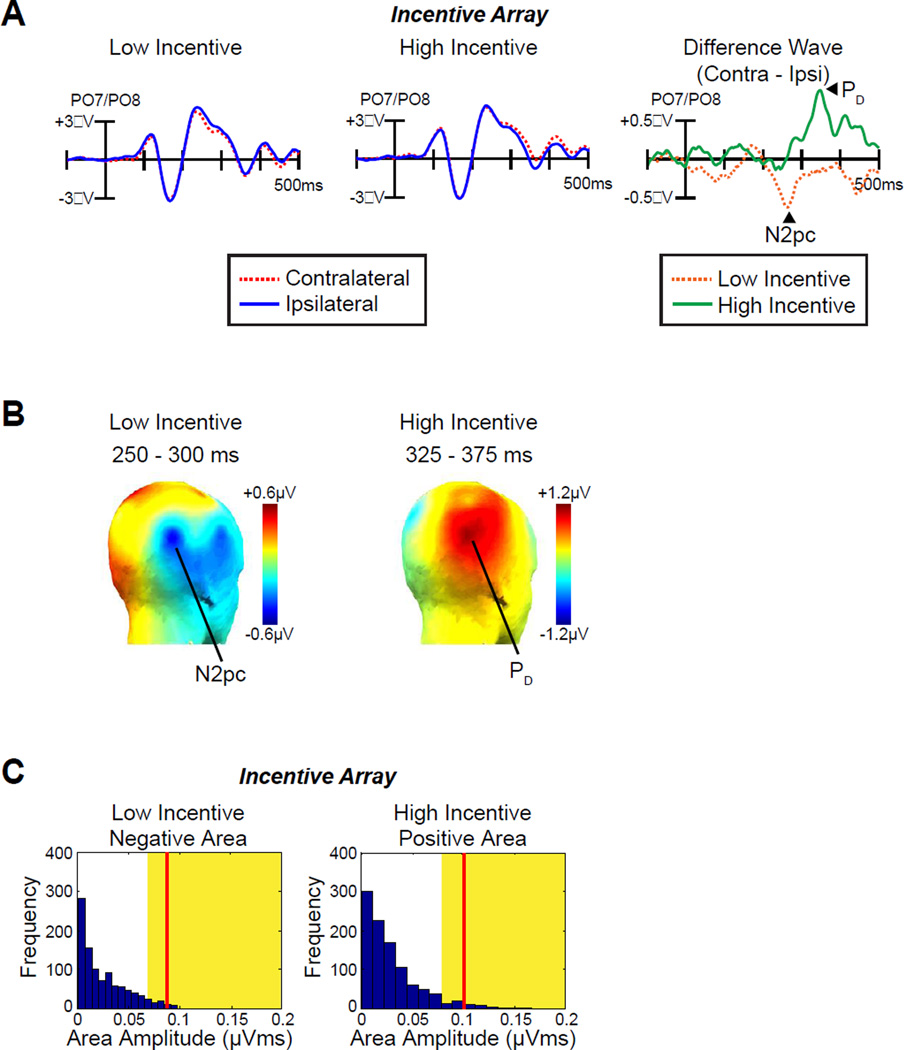
Fig. 2A shows the ERP waveforms triggered by the incentive array as measured at lateral occipital-temporal electrodes (PO7/PO8) contralateral and ipsilateral to the incentive cues. An N2pc component (a contralateral negativity) was observed on low-incentive trials, indicating that attention was allocated to the incentive cue on these trials. However, a PD component (a contralateral positivity) was observed on high-incentive trials, indicating a suppression of the cue item on these trials. This is what would be expected if participants tried to avoid wasting cognitive resources on the incentive cue so that they could prepare for the target on high-incentive trials. Note that no significant laterality effects including contralateral delay activity (CDA) were observed beyond 500 ms. Topographic maps of the N2pc and PD components are plotted in Fig 2B.
Fig 2.
ERP results from incentive array. A, Grand average waveforms for low- and high-incentive cues at contralateral versus ipsilateral electrode sites (averaged over PO7 and PO8). The time 0 is the onset of incentive array. B, Topographic maps of the N2pc component (low-incentive cue) and the PD component (high-incentive cue). C, Permutation tests of the negative area for low-incentive cues and positive area for high-incentive cues from 100–500 ms. The blue bars indicate the estimated null distribution from 1000 permutations. The yellow areas indicate the top 5% of the permutation distribution. The red lines represent the observed values of the negative area (N2pc, low-incentive cues) and positive area (PD, high-incentive cues) from the grand average difference waveforms. Because the red lines fall within the yellow regions, the observed values are significantly greater than would be expected by chance.
The voltage was significantly different from zero in one-sample t-test of the contralateral-ipsilateral difference waves for both the N2pc component on low-incentive trials [time-window: 250–300 ms, t (12) = −2.2, p = .048] and the PD component on high-incentive trials [time-window: 325–375 ms, t (12) = 3.5, p = .005]. Although conventional, this method of analysis used measurement intervals that were selected on the basis of the effects observed in the waveforms, possibly biasing outcomes (see Luck, 2014). We therefore repeated the analyses with a permutation approach (see Methods) that does not require specifying a narrow measurement window. These analyses confirmed that the PD and N2pc were significantly different from chance (p = .023 and p = .007, respectively, Fig. 2C).
Preparation Interval TFR
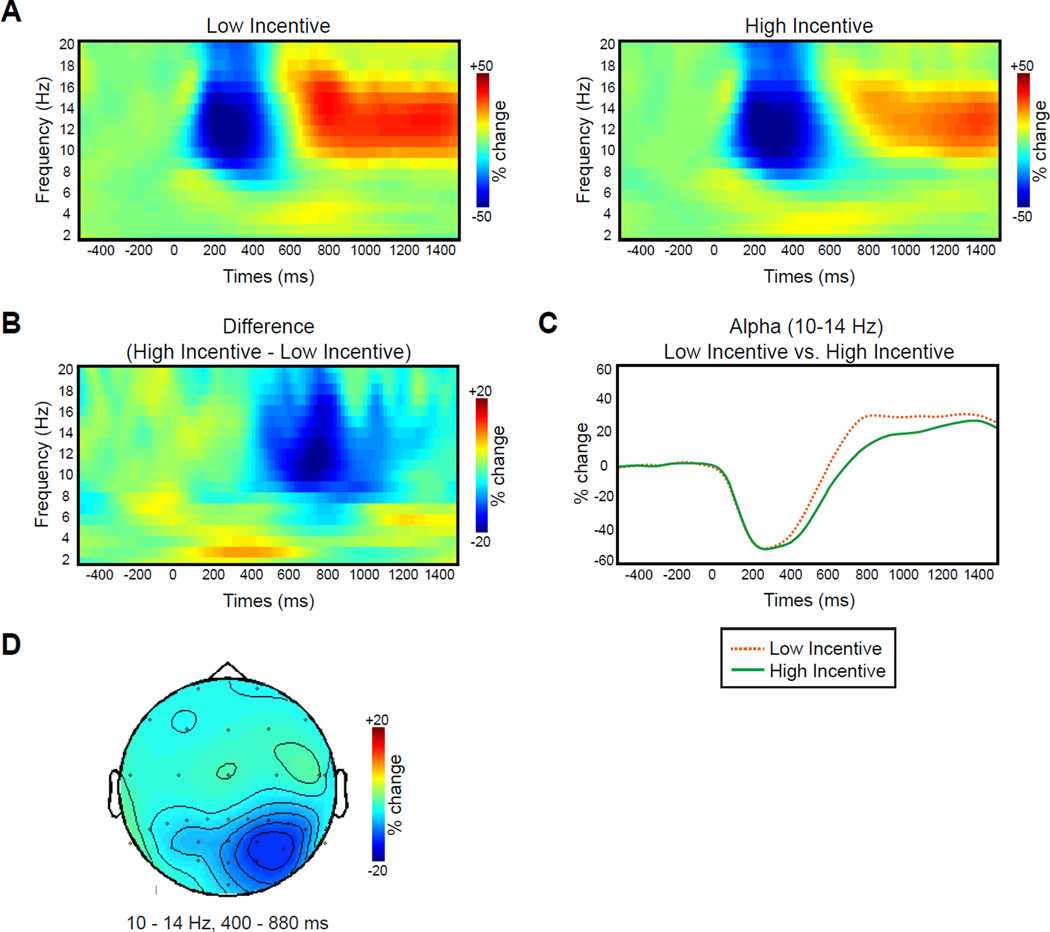
Fig. 3 shows the TFRs of the data following the incentive cue arrays. For both low- and high-incentive cue trials, power in the alpha frequency range (10–14 Hz) decreased from approximately 200–400 ms and then increased from approximately 600 ms until the end of the preparation interval (Fig. 3A). However, the increase in alpha activity in the latter part of the interval was substantially smaller following high-incentive cues than following low-incentive cues (Fig. 3B and C). A cluster randomization analysis revealed a significant difference between these conditions at a cluster of parietal-occipital electrode sites (centered near the PO4 site) from 400 to 880 ms. This is exactly what would be expected if participants adopted a greater visual readiness state following the high-incentive cues than following the low-incentive cues.
Fig 3.
TFR results during preparation interval. A. Grand average power of neural oscillations in the EEG plotted as a function of oscillation frequency and time after the onset of the incentive array (and before the search array appeared) for low- and high-incentive trials at PO4. B. Grand average of the difference TFRs of power (high- minus low-incentive trials) at PO4. C. Grand average % change in power at 10–14 Hz for low- and high-incentive trials at PO4. D. Grand average topography of the difference TFRs of power (high minus low-incentive trials) averaged over 10–14 Hz and 400–880 ms.
Visual Search Array ERPs
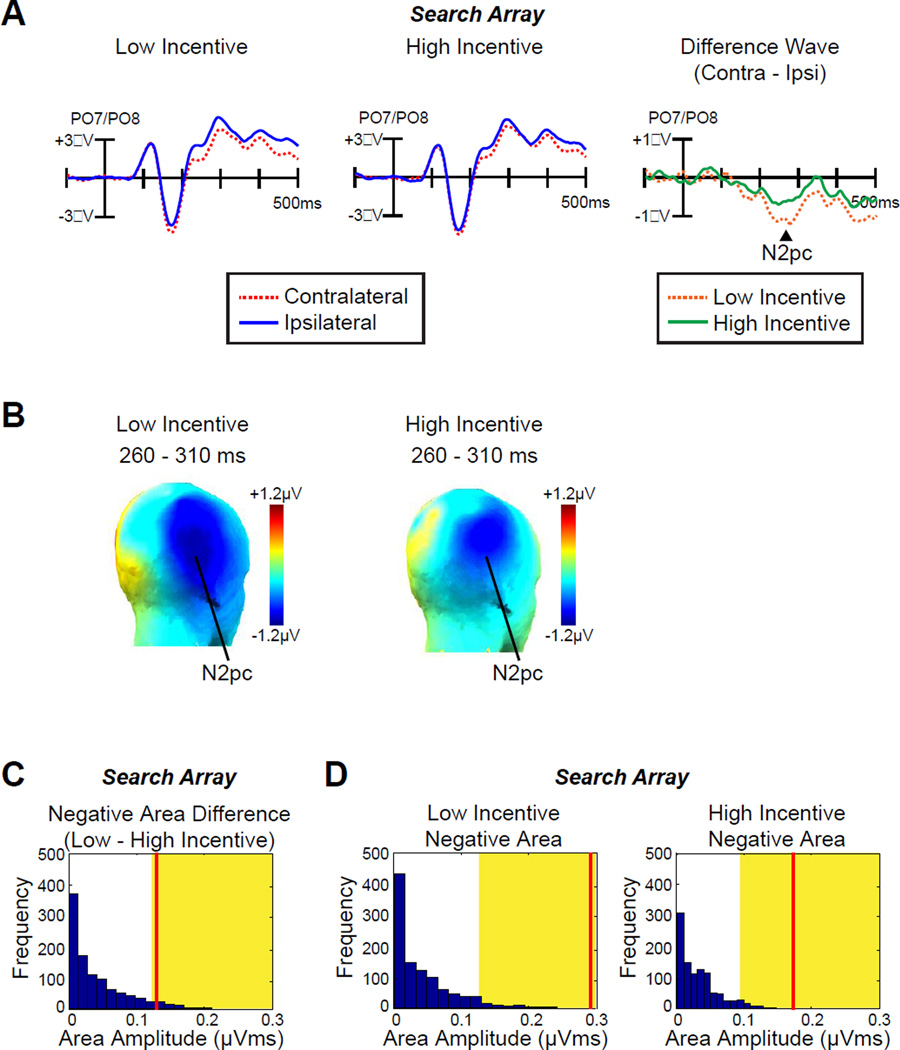
Fig. 4A shows that the N2pc for visual search targets was approximately half as large on high-incentive trials as on low-incentive trials (topographic maps of the N2pc component are plotted in Fig 4B). This was a significant difference [paired-sample t-test; t (12) = −2.2, p = .046, time-window: 260–310 ms]. A permutation test confirmed that the observed N2pc (negative area) difference between low- and high-incentive trials was significantly greater than would be expected by chance (p = .047, Fig.4C). The N2pc was only marginally significantly different from zero in the high-incentive condition [one-sample t-test: t (12) = −1.9, p = .076] but was highly significantly different from zero in the low-incentive condition [t (12) = −5.3, p < .001]. Permutation tests indicated that the voltages were beyond the chance level for both conditions (p < .001 for both, Fig. 4D). Thus, increased incentives led the participants to discriminate the target rapidly and accurately with reduced attentional allocation.
Fig 4.
ERP results from search array. A, Grand average waveforms for low- and high-incentive targets at contralateral versus ipsilateral electrode sites (averaged over PO7 and PO8). The time 0 is the onset of search array. B, Topographic maps of the N2pc component for low- and high-incentive targets. C, Permutation tests of the negative areas difference between low- and high-incentive targets from 100–500 ms. The blue bars indicate the estimated null distribution from 1000 permutations. The yellow areas indicate the top 5% of the permutation distribution. The red lines represent the observed values of the negative area (N2pc) difference between low- and high-incentive targets from the grand average difference waveforms. D, Permutation tests of the negative areas for low- and high-incentive targets from 100–500 ms. The red lines represent the observed values of the negative area (N2pc) from the grand average difference waveforms.
DISCUSSION
This study provided a clear demonstration that humans are able to use cues signaling the imminent availability of large rewards to enhance the efficiency of selective attention on a subsequent task. Although it might appear straightforward that incentives should enhance performance, there are two reasons why this behavioral effect is noteworthy. First, prior studies reporting performance enhancement on visual search tasks (Engelmann & Pessoa, 2007; Small et al., 2005) provided participants with an opportunity to prepare a specific motor response as well as attentional responses, leading to the possibility that motivation enhances only motor preparation, without influencing selective attention (Mir et al., 2011). In the present study, however, a specific motor response could not be prepared because the incentive cue did not predict which response would be appropriate for the subsequent task, and yet a robust effect of motivation was observed. Second, because points were awarded for correct responses on both low- and high-incentive trials, observers could have ignored the incentive display completely, resulting in the same level of performance regardless of incentive. Instead, responses were faster on high-incentive trials. The behavioral benefit of high incentives relative to low incentives shows that incentives can facilitate premotor visual search processes. Interestingly, search errors were not more likely with high versus low incentives, bolstering the notion that motivation enhances cognitive efficiency rather than simply speeding motor behavior.
Even stronger evidence for an effect on attentional processes was provided by the EEG/ERP data. These data provide three specific insights into the sequence of processes that were triggered by high-incentive cues. First, in the ERP elicited by the incentive display, we found a significant PD component when the incentive was high and a significant N2pc component when the incentive was low. Thus, observers suppressed high-incentive cues. Second, during the preparation interval (after the incentive display and before the search array) we observed lower alpha power following high relative to low incentives, reflecting heightened visual readiness. Finally, in the ERP elicited by the search array, we found that the N2pc component was smaller on high-incentive trials than on low-incentive trials, just as the N2pc is smaller for easy search tasks than for more difficult search tasks.
The finding of a PD to the high-incentive cue is a particularly counterintuitive result, because prior research suggests that reward-associated stimuli automatically capture attention (Anderson et al., 2011). Indeed, we did observed attention capture (an N2pc) to the low-incentive cue. However, this previous research also shows that the automatic capture of attention by reward-related stimuli can be deleterious to task performance, and other research shows that active suppression (indexed by PD) can be used to avoid shifting attention to salient but potentially distracting stimuli (Gaspar & McDonald, 2014; Sawaki & Luck, 2010). In the present study, the high-, low- and non-informative cues gained different levels of stimulus saliency through the learning task and attentional priorities of those cues differed with respect to their learned stimulus saliency. Therefore, when the high-incentive and non-informative cues were presented on the incentive array, the high-incentive cue had a higher attentional priority than the non-informative cues. However, the cue did not predict the location of the upcoming target or a correct response and thus a strong focus of attention toward it could have interfered with performing the task. Consequently, participants may have actively suppressed the high-incentive cues to avoid being distracted by them. Finding an N2pc instead of a PD for low-incentive cues suggests that suppression is somehow costly and worth the additional effort only when the payoff is likely to be high. This is consistent with previous research (Kiss et al., 2011) showing that attention is deployed toward color singleton distractors when temporal task demands are low so that allocating attention toward distractors does not compromise successful target detection. In contrast, suppression is observed when temporal task demands are high and thus directing attention toward distractors could result in missing targets. Therefore, it is possible that high-incentive cues could elicit a large N2pc if a strong focus of attention toward them did not interfere with performing the task. One might question how participants could use the high incentive cue to facilitate performance if they had not attended it. However, it is well established that simple features can be detected even when spatial attention has not been focused on them (as indexed by the lack of an N2pc) (Burra & Kerzel, 2014; Luck & Ford, 1998).
The presentation of high versus low incentives continued to impact processing after the incentive array, with reduced alpha activity after high- relative to low-incentive cues during the middle third of the interval. Decreased alpha power is thought to reflect greater readiness to process external stimuli (Mazaheri et al., 2014, 2009). Our results suggest that motivational processes initiated by the incentive display were able to influence perceptual readiness in advance of the visual search array. This effect was mainly observed at right-hemisphere parietal-occipital electrode sites (Fig. 3D), which is consistent with prior work on readiness (Thiel & Fink, 2007).
There were two likely ways in which incentives might facilitate attention to the target: more attention would be allocated on high-incentive trials (yielding a larger N2pc on high- versus low-incentive trials), or attentional processing would become more efficient on high-incentive trials, which would reduce the need for focused attention (yielding a smaller N2pc on high- versus low-incentive trials). Our finding of reduced N2pc amplitude on high-incentive trials suggests that processing was more efficient on these trials, which is consistent with previous research showing that (a) the N2pc is reduced for simple targets when participants are motivated to do the search task with minimal attention (Luck & Ford, 1998) and (b) the N2pc is smaller in tasks that are less attention-demanding (Anderson et al., 2014; Burra & Kerzel, 2014; Eimer, 1996; Luck & Hillyard, 1994b). It has been proposed that heightened readiness allows attentional mechanisms to take better advantage of cognitive preparation by enhancing the motivational salience (i.e., the motivational significance) of the target (Bromberg-Martin, Matsumoto, & Hikosaka, 2010), and such competitive stimuli require less attentional allocation to be selected. Note that high-incentive trials also produced faster RTs, showing that the smaller N2pc on these trials cannot be explained by a failure of target selection. Interestingly our finding of a smaller N2pc under more motivated conditions contrasts with prior results (Kiss et al., 2009) showing a larger N2pc for reward-associated targets. However, the reward value of the target in this task could not be predicted prior to the onset of the target, precluding differential perceptual preparation. In other words, this previous study manipulated the magnitude of the actual reward rather cuing the reward associated with an upcoming stimulus.
Fluctuations in the efficiency of cognitive processing are commonly experienced, yet how they occur and whether we can control them remains poorly understood. The present study provides a first step toward outlining the sequence of neural operations that allow cognitive processing to be sharpened “on the fly” in response to information that signals an upcoming reward opportunity. This has important implications for understanding human performance in many situations that involve incentives, ranging from sports to social interactions.
ACKNOWLEDGEMENTS
This research was made possible, in part, by NIH grant MH076226 to S.J.L., ESRC grant ES/L001210/1 to J.E.R., and JSPS postdoctoral fellowship to R.S.
REFERENCE
- American Electroencephalographic Society. Guideline thirteen: guidelines for standard electrode position nomenclature. Journal of Clinical Neurophysiology. 1994;11:111–113. [PubMed] [Google Scholar]
- Anderson BA, Laurent PA, Yantis S. Value-driven attentional capture. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:10367–10371. doi: 10.1073/pnas.1104047108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DE, Ester EF, Klee D, Vogel EK, Awh E. Electrophysiological evidence for failures of item individuation in crowded visual displays. Journal of Cognitive Neuroscience. 2014;26:2298–2309. doi: 10.1162/jocn_a_00649. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Berridge KC. From prediction error to incentive salience: mesolimbic computation of reward motivation. The European Journal of Neuroscience. 2012;35:1124–1143. doi: 10.1111/j.1460-9568.2012.07990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68:815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Goodman J, Burock M, Rotte M, Koutstaal W, Schacter D, Dale AM. Functional-anatomic correlates of object priming in humans revealed by rapid presentation event-related fMRI. Neuron. 1998;20:285–296. doi: 10.1016/s0896-6273(00)80456-0. [DOI] [PubMed] [Google Scholar]
- Burra N, Kerzel D. The distractor positivity (Pd) signals lowering of attentional priority: Evidence from event-related potentials and individual differences. Psychophysiology. 2014;51:685–696. doi: 10.1111/psyp.12215. [DOI] [PubMed] [Google Scholar]
- Cohen MX. Analyzing Neural Time Series Data: Theory and Practice. Cambridge: MIT Press; 2014. [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Eimer M. The N2pc component as an indicator of attentional selectivity. Electroencephalography and Clinical Neurophysiology. 1996;99:225–234. doi: 10.1016/0013-4694(96)95711-9. [DOI] [PubMed] [Google Scholar]
- Engelmann JB, Pessoa L. Motivation sharpens exogenous spatial attention. Emotion. 2007;7:668–674. doi: 10.1037/1528-3542.7.3.668. [DOI] [PubMed] [Google Scholar]
- Ernst MD. Permutation Methods: A Basis for Exact Inference. Statistical Science. 2004;19:676–685. [Google Scholar]
- Gaspar JM, McDonald JJ. Suppression of salient objects prevents distraction in visual search. The Journal of Neuroscience. 2014;34:5658–5666. doi: 10.1523/JNEUROSCI.4161-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RNA. Neuroimaging studies of priming. Progress in Neurobiology. 2003;70:53–81. doi: 10.1016/s0301-0082(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Hickey C, Chelazzi L, Theeuwes J. Reward changes salience in human vision via the anterior cingulate. The Journal of Neuroscience. 2010;30:11096–11103. doi: 10.1523/JNEUROSCI.1026-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey C, Di Lollo V, McDonald JJ. Electrophysiological indices of target and distractor processing in visual search. Journal of Cognitive Neuroscience. 2009;21:760–775. doi: 10.1162/jocn.2009.21039. [DOI] [PubMed] [Google Scholar]
- Hopf JM, Boelmans K, Schoenfeld MA, Luck SJ, Heinze HJ. Attention to features precedes attention to locations in visual search: evidence from electromagnetic brain responses in humans. The Journal of Neuroscience. 2004;24:1822–1832. doi: 10.1523/JNEUROSCI.3564-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf JM, Luck SJ, Boelmans K, Schoenfeld MA, Boehler CN, Rieger J, Heinze HJ. The neural site of attention matches the spatial scale of perception. The Journal of Neuroscience. 2006;26:3532–3540. doi: 10.1523/JNEUROSCI.4510-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokisch D, Jensen O. Modulation of gamma and alpha activity during a working memory task engaging the dorsal or ventral stream. The Journal of Neuroscience. 2007;27:3244–3251. doi: 10.1523/JNEUROSCI.5399-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung TP, Makeig S, Westerfield M, Townsend J, Courchesne E, Sejnowski TJ. Removal of eye activity artifacts from visual event-related potentials in normal and clinical subjects. Clinical Neurophysiology. 2000;111:1745–1758. doi: 10.1016/s1388-2457(00)00386-2. [DOI] [PubMed] [Google Scholar]
- Kiss M, Driver J, Eimer M. Reward priority of visual target singletons modulates event-related potential signatures of attentional selection. Psychological Science. 2009;20:245–251. doi: 10.1111/j.1467-9280.2009.02281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lins OG, Picton TW, Berg P, Scherg M. Ocular artifacts in EEG and event-related potentials I : scalp topography. Brain Topography. 1993;6:51–63. doi: 10.1007/BF01234127. [DOI] [PubMed] [Google Scholar]
- Lopez-Calderon J, Luck SJ. ERPLAB: an open-source toolbox for the analysis of event-related potentials. Frontiers in Human Neuroscience. 2014;8:213. doi: 10.3389/fnhum.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ. Electrophysiological correlates of the focusing of attention within complex visual scenes: N2pc and related ERP components. In: Luck SJ, Kappenman ES, editors. Oxford handbook of ERP components. New York: Oxford University Press; 2012. pp. 329–360. [Google Scholar]
- Luck SJ. An introduction to the event-related potential technique. Cambridge: MIT Press; 2014. [Google Scholar]
- Luck SJ, Ford MA. On the role of selective attention in visual perception. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:825–830. doi: 10.1073/pnas.95.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA. Electrophysiological correlates of feature analysis during visual search. Psychophysiology. 1994a;31:291–308. doi: 10.1111/j.1469-8986.1994.tb02218.x. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA. Spatial filtering during visual search: evidence from human electrophysiology. Journal of Experimental Psychology. Human Perception and Performance. 1994b;20:1000–1014. doi: 10.1037//0096-1523.20.5.1000. [DOI] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. Journal of Neuroscience Methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Mazaheri A, Nieuwenhuis ILC, van Dijk H, Jensen O. Prestimulus alpha and mu activity predicts failure to inhibit motor responses. Human Brain Mapping. 2009;30:1791–1800. doi: 10.1002/hbm.20763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaheri A, van Schouwenburg MR, Dimitrijevic A, Denys D, Cools R, Jensen O. Region-specific modulations in oscillatory alpha activity serve to facilitate processing in the visual and auditory modalities. NeuroImage. 2014;87:356–362. doi: 10.1016/j.neuroimage.2013.10.052. [DOI] [PubMed] [Google Scholar]
- Mir P, Trender-Gerhard I, Edwards MJ, Schneider SA, Bhatia KP, Jahanshahi M. Motivation and movement: the effect of monetary incentive on performance speed. Experimental Brain Research. 2011;209:551–559. doi: 10.1007/s00221-011-2583-5. [DOI] [PubMed] [Google Scholar]
- O’Brien JL, Raymond JE. Learned predictiveness speeds visual processing. Psychological Science. 2012;23:359–363. doi: 10.1177/0956797611429800. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational Intelligence and Neuroscience. 2011;2011:156869. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond JE, O’Brien JL. Selective visual attention and motivation: the consequences of value learning in an attentional blink task. Psychological Science. 2009;20:981–988. doi: 10.1111/j.1467-9280.2009.02391.x. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Heise KF, Gruber WR, Holz E, Karim AA, Hummel FC. Brain oscillatory substrates of visual short-term memory capacity. Current Biology. 2009;19:1846–1852. doi: 10.1016/j.cub.2009.08.062. [DOI] [PubMed] [Google Scholar]
- Sawaki R, Geng JJ, Luck SJ. A common neural mechanism for preventing and terminating the allocation of attention. The Journal of Neuroscience. 2012;32:10725–10736. doi: 10.1523/JNEUROSCI.1864-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaki R, Luck SJ. Capture versus suppression of attention by salient singletons: electrophysiological evidence for an automatic attend-to-me signal. Attention, Perception & Psychophysics. 2010;72:1455–1470. doi: 10.3758/APP.72.6.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaki R, Luck SJ. Active suppression after involuntary capture of attention. Psychonomic Bulletin & Review. 2013;20:296–301. doi: 10.3758/s13423-012-0353-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM, Gitelman D, Simmons K, Bloise SM, Parrish T, Mesulam MM. Monetary incentives enhance processing in brain regions mediating top-down control of attention. Cerebral Cortex. 2005;15:1855–1865. doi: 10.1093/cercor/bhi063. [DOI] [PubMed] [Google Scholar]
- Thiel CM, Fink GR. Visual and auditory alertness: modality-specific and supramodal neural mechanisms and their modulation by nicotine. Journal of Neurophysiology. 2007;97:2758–2768. doi: 10.1152/jn.00017.2007. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Luck SJ. Serial deployment of attention during visual search. Journal of Experimental Psychology. Human Perception and Performance. 2003;29:121–138. doi: 10.1037//0096-1523.29.1.121. [DOI] [PubMed] [Google Scholar]