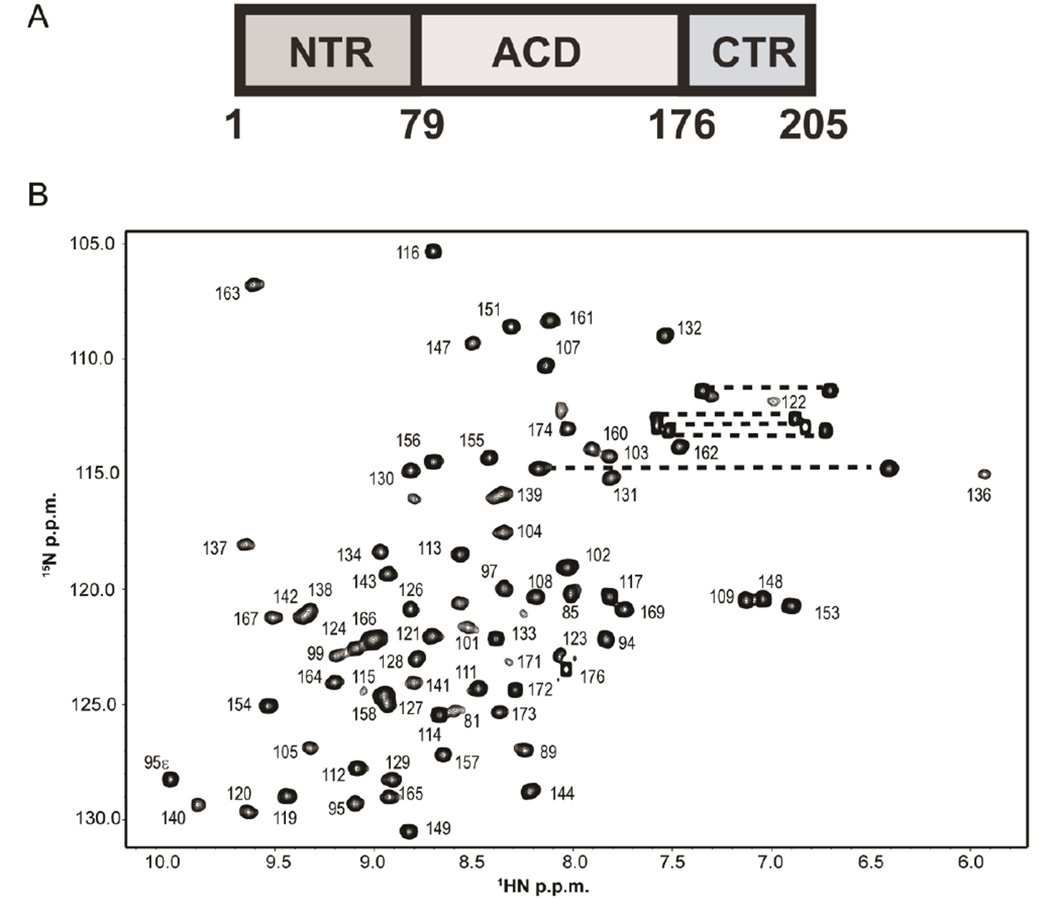
Fig. 1.
HSPB1 contains a central α-crystallin domain that is necessary and sufficient for dimer formation A) Domain organization of human HSPB1. The 100 α-crystallin domain (ACD) is flanked by a 78-residue N-terminal region (NTR) and a 29-residue C-terminal region (CTR). In the absence of the NTR and CTR, the ACD forms a homodimer in solution. B) 1H-15N HSQC spectrum of HSPB1-ACD with assignments. Dashed lines connect side-chain amide groups of Gln and Asn residues. The spectrum was acquired at 800 MHz at 22 °C on a 0.5 mM sample in sodium phosphate buffer (pH 7.5) containing 100 mM NaCl.