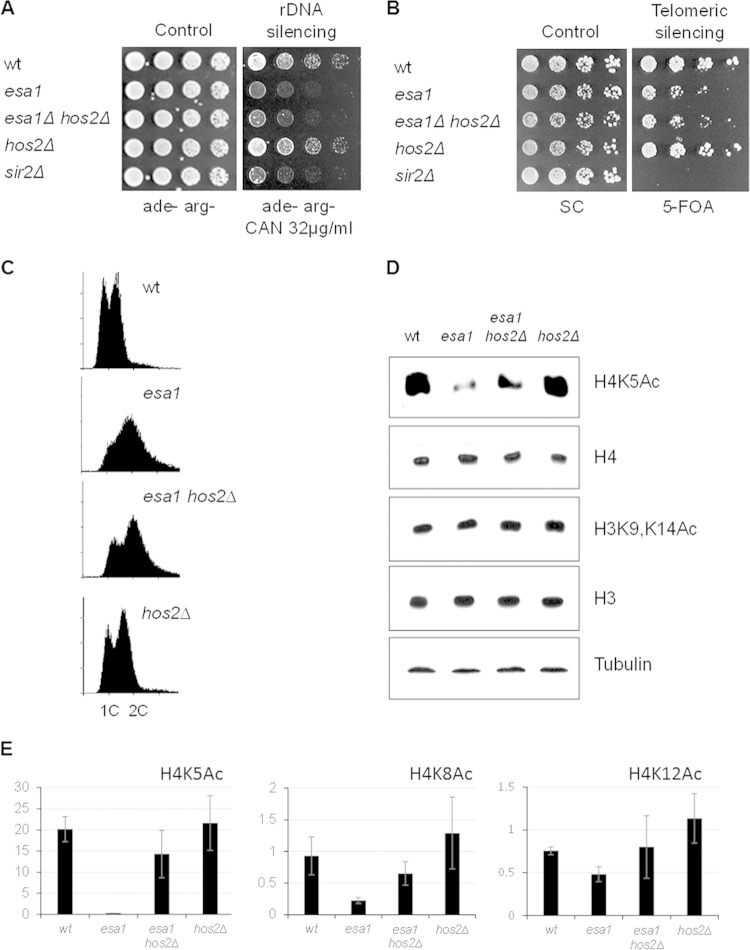
FIG 2.
Deleting HOS2 improved histone H4 acetylation in esa1 but did not restore silencing and cell cycle regulation. (A) The esa1 hos2Δ strain is defective for rDNA silencing. The wild-type (LPY4908), esa1-414 (LPY4912), esa1-414 hos2Δ (LPY18074), hos2Δ (LPY18073), and sir2Δ (LPY5015) strains carry the rDNA::ADE2-CAN1 reporter. Defective rDNA silencing leads to expression of CAN1 and sensitivity to canavanine. (B) The esa1 hos2Δ strain had telomeric silencing defects. The wild-type (LPY4916), esa1-414 (LPY13520), esa1-414 hos2Δ (LPY18070), hos2Δ (LPY18071), and sir2Δ (LPY5034) strains carry the TELVR::URA3 reporter. Defective telomeric silencing results in 5-FOA sensitivity. (C) Cell cycle profiles showed a significant G2/M delay in cell cycle progression at 30°C in esa1-531 (LPY14757) cells that was modestly improved in the esa1-531 hos2Δ (LPY14761) strain. Control strains were the wild-type (LPY6497) and hos2Δ (LPY14577) strains. (D) Deletion of HOS2 increased acetylation of histone H4K5 in esa1 mutants. H3K9 and K14 acetylation was unaffected by mutation of ESA1 or hos2Δ. Whole-cell protein lysates from strains in panel C were immunoblotted as noted. The experiment was also performed with esa1-414 strains, with similar results. (E) Deletion of HOS2 improved acetylation of other H4 lysines in esa1 strains. Quantification of histone H4 acetylation at K5, K8, and K12 relative to histone levels was performed using two to four independent Western blots. ImageQuant 5.2 (Molecular Dynamics) and the histogram peak function were used as for Fig. 1. Representative immunoblots are shown in Fig. S2 in the supplemental material.