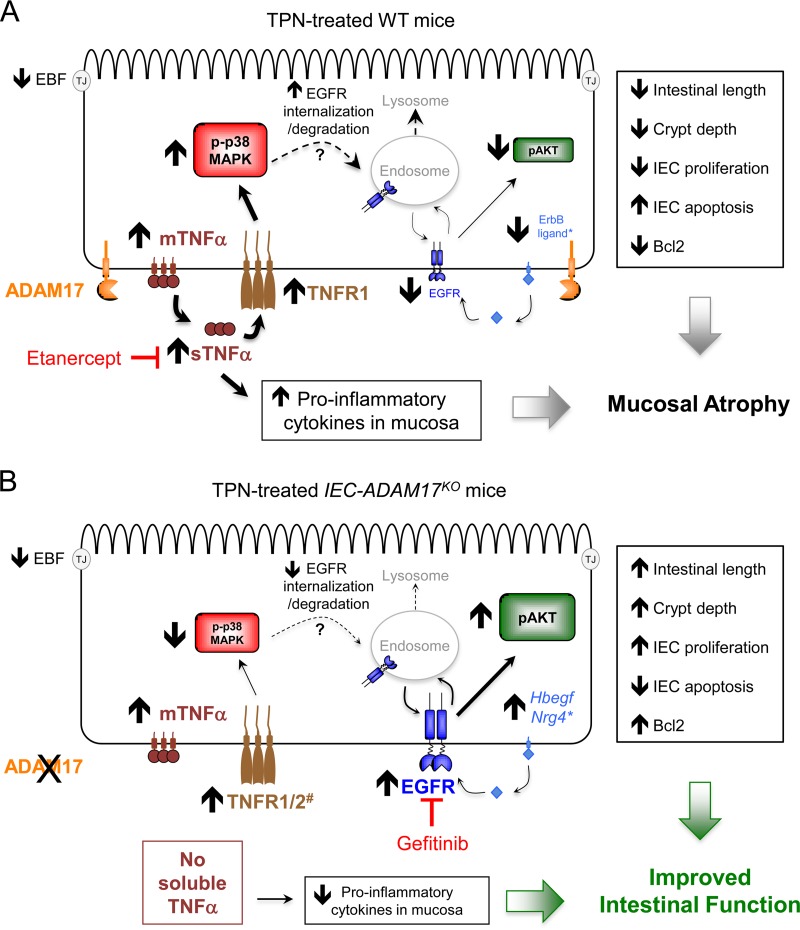
FIG 11.
Model for ADAM17-mediated TNF-α signaling in IECs and the development of TPN-induced mucosal atrophy. (A) In TPN-treated wild-type (WT) mice, the TNF-α and TNFR1 mRNA and protein levels are upregulated in IECs, leading to increased production of soluble TNF-α, increased TNFR1 signaling, and activation of p-p38-MAPK in IECs. There is a concomitant reduction in EGFR protein levels and decreased functional pAKT signaling in IECs. *, EGFR signaling is further compromised by reduced ErbB ligand mRNA expression within the intestinal mucosa. Etanercept blocks soluble TNF-α signaling, leading to reduced p38-MAPK signaling in IECs. There is a concomitant restoration of EGFR protein levels and functional pAKT signaling in IECs. (B) In TPN-treated IEC-ADAM17KO mice, TNF-α and TNFR1 mRNA and protein levels are upregulated in IECs. However, there is no generation of soluble TNF-α leading to reduced TNFR1 and p-p38-MAPK signaling in IECs. #, ADAM17 deficiency also increases TNFR2 cell surface protein levels, which may alter the balance of TNFR signaling in IECs. Despite the loss of ADAM17-mediated ErbB ligand shedding in IECs, there is a partial restoration of EGFR protein levels and functional pAKT signaling in IECs. *, increased Hbegf and Nrg4 mRNA levels in the intestinal mucosa may contribute to this process. Gefitinib blocks these protective effects confirming the importance of functional EGFR signaling in the attenuation of mucosal atrophy observed in TPN-treated IEC-ADAM17KO mice.