Abstract
Extant deep-sea invertebrate fauna represent both ancient and recent invasions from shallow-water habitats. Hydrostatic pressure may present a significant physiological challenge to organisms seeking to colonize deeper waters or migrate ontogenetically. Pressure may be a key factor contributing to bottlenecks in the radiation of taxa and potentially drive speciation. Here, we assess shifts in the tolerance of hydrostatic pressure through early ontogeny of the northern stone crab Lithodes maja, which occupies a depth range of 4–790 m in the North Atlantic. The zoea I, megalopa and crab I stages were exposed to hydrostatic pressures up to 30.0 MPa (equivalent of 3000 m depth), and the relative fold change of genes putatively coding for the N-methyl-d-aspartate receptor-regulated protein 1 (narg gene), two heat-shock protein 70 kDa (HSP70) isoforms and mitochondrial Citrate Synthase (CS gene) were measured. This study finds a significant increase in the relative expression of the CS and hsp70a genes with increased hydrostatic pressure in the zoea I stage, and an increase in the relative expression of all genes with increased hydrostatic pressure in the megalopa and crab I stages. Transcriptional responses are corroborated by patterns in respiratory rates in response to hydrostatic pressure in all stages. These results suggest a decrease in the acute high-pressure tolerance limit as ontogeny advances, as reflected by a shift in the hydrostatic pressure at which significant differences are observed.
Keywords: deep sea, evolution, species radiation, Lithodidae, physiological bottleneck, hydrostatic pressure
1. Introduction
Many deep-sea invertebrates are thought to have evolved in shallow habitats and radiated into deep waters (defined as depths below 200 m) [1], although re-emergence into shallow waters is also reported [2,3]. Periods of climate-driven extinction and replacement are thought to define evolution in the deep sea, with evidence for successive cycles of shallow-water taxa invading the deep sea throughout the Phanerozoic [4–6]. Evolutionary patterns may be influenced by global anoxic and dysoxic conditions driving extinction events in this environment [6,7], with the most severe extinction events occurring in the mid-Cretaceous, and at the Permian–Triassic and Ordovician–Silurian boundaries [4]. Some deep-sea taxa appear to have escaped extinction, undergoing extensive in situ radiation and diversification [8,9]. Extant deep-sea taxa are therefore thought to consist of both ancient and recent lineages with shallow-water origins.
Benthic deep-sea fauna show distinct zonation patterns, with the highest levels of species turnover found at the upper slope boundary (approx. 1000 m) and at the transition zone between the slope and the abyssal plain (2000–3000 m) [10], indicating biodiversity bottlenecks [11]. Depth is an important predictor of diversity, and unimodal trends in biodiversity at bathyal depths suggest that these depths are the primary site of speciation in the deep sea [12]. The depth-differentiation hypothesis proposes that speciation is encouraged by the large temporal and spatial environmental heterogeneity found at bathyal depths [13]. While distributional limits may be attributed to a number of biotic and abiotic factors, including competition, topographic complexity and population isolation due to oxygen minimum zones, the physiological effects of low temperature and high pressure are also suggested to play an important role in determining vertical structure, limiting gene flow and driving speciation by promoting a stress-evolution mechanism [5,6,10,12–15]. Studies testing the hyperbaric tolerance of shallow-water invertebrates at ecologically relevant temperatures consistently find evidence for a common hydrostatic pressure threshold at bathyal depths [14].
Hydrostatic pressure affects many levels of biological organization, including behavioural, cellular and molecular responses [14,16]. At a molecular and structural level, pressure is known to affect lipid bilayer fluidity, enzyme processes and protein folding in shallow-water adapted organisms [17–19]. High pressures and low temperatures both cause membrane lipids to become more ordered, moving from a fluid phase to a gel phase [20]. Such changes have knock-on effects at other levels of biological hierarchy. For example, changes in membrane fluidity affect a number of membrane-associated processes, including those involved in cellular signalling [21].
Invertebrate and vertebrate species share a common, generic stress response to multiple potential environmental stressors, known as the cellular stress response (CSR), which includes the activation and upregulation of a set of highly conserved stress proteins that assess and counteract macromolecular damage [22]. In marine systems, transcriptome-based approaches are increasingly being applied to monitor the CSR and apply thresholds for phenotypic plasticity, to assess the capacity for organisms to modify their phenotype to adapt to new environmental conditions [23–25]. Previous investigations of molecular physiological responses to temperature and pressure in invertebrates have focused on generic markers of stress and the CSR members of the heat-shock protein 70 kDa family (HSP70) [26–28], but more recently, this has been extended to novel pressure-specific stress markers [29].
Anomuran crabs of the family Lithodidae represent a case of speciation and range expansion through global cold waters. Lithodids are thought to have evolved in the northeast Pacific from a shallow-water anomuran population in the Cenozoic era (approx. 13–25 Mya), and consist of two subfamilies: the Haplogastrinae and Lithodinae [2,30]. The Haplogastrinae and some Lithodinae radiated within the shallow north Pacific; however, three lithodine genera (Lithodes, Neolithodes and Paralomis) colonized deep water before radiating and extending their distribution into other ocean basins [2]. Bottlenecks in the radiation of the Lithodidae have so far been assigned to lineage-specific thermal tolerances and differences in early life histories among genera [2].
Lithodes maja and Lithodes santolla, currently found in the Atlantic, are thought to have originated from among the earliest lithodids to leave the north Pacific [31]. L. maja occupies ‘intermediate’ depths relative to its confamilials (4–790 m), and appears to have extended its bathymetric distribution in order to colonize the north Atlantic [31,32]. As with other lithodines, the larval development of L. maja is lecithotrophic and involves morphological and metabolic reorganization during early development, passing through three putatively demersal planktonic zoeal stages and a megalopa stage, which settles and metamorphoses into the juvenile crab I stage (the first feeding stage) [32–35].
This paper investigates the effects of hydrostatic pressure on respiration rate, and the transcription of four genes in three distinct early ontogenetic stages (zoea I, megalopa, crab I) of the northern stone crab, L. maja. This study uses traditional markers of the CSR (HSP70 isoforms, hsp70a, hsp70b genes), a marker of aerobic capacity (Citrate synthase, CS gene), and a potential pressure-specific marker (narg gene, putatively coding for N-methyl-d-aspartate receptor-regulated protein 1) to test the hypothesis that hydrostatic pressure tolerance decreases with increasing ontogenetic age. Members of the HSP70 family are not only involved in protein folding/unfolding, assembly/disassembly and the degradation of misfolded proteins under ‘normal’ non-stress conditions, but also play an important role in stress resistance [36]. The nuclear CS gene encodes the citrate synthase enzyme; CS enzyme activity is considered a correlate of aerobic capacity at a mitochondrial level and is used as an indicator of aerobic metabolism [37–41]. N-methyl-d-aspartate (NMDA) receptors are thought to play an important role in the onset of high-pressure neurological syndrome (HPNS) in vertebrates, which is characterized by a series of consistent pathologies, including convulsions [42–44]. HPNS occurs as a result of hyper-excitability of the central nervous system, and studies in rats and the frog Xenopus laevis support the hypothesis that it is characterized in part by an increase in the sensitivity of the NMDA receptor [45,46]. By quantifying expression of these genes, we are able to examine the molecular physiological constraints acting on the early life stages of L. maja, and the degree to which acute changes in hydrostatic pressure might constrain bathymetric distributions at each ontogenetic stage.
2. Material and methods
(a). Adult sampling and maintenance
Adult specimens of L. maja were collected during September and October 2011 using baited traps in Gullmarsfjord, Sweden, at depths of approximately 60 m (approximate location: 58°21′0″ N, 11°34′48″ E). Before relocation to the National Oceanography Centre Southampton (NOCS), UK, the animals were maintained for between 2 and 6 weeks in an open aquarium system (temperature approx. 10°C, natural light cycle, atmospheric pressure) at the Sven Lovén Centre for Marine Sciences, Kristineberg, Sweden, in seawater from a 32 m deep intake. The adults were transferred to Southampton in a temperature-controlled van at 6°C, and travel time was less than 24 h. The animals were starved for several days prior to transportation and were transported individually in polystyrene boxes lined with wetted towels. Subsequently, animals were maintained in a recirculating aquarium (temperature 6°C, 24 h darkness, atmospheric pressure) at NOCS. The maintenance temperature was chosen to match the temperature in the field.
(b). Larval rearing
Larvae were obtained from two gravid females. Individuals used for gene expression studies were obtained from a single female (pre-moult carapace 63.4 mm; post-moult carapace length 70.1 mm) that moulted during the night of 3 April 2012 and oviposited the following day. Although the use of offspring from a single female may result in reduced genetic variation between individuals, the lengthy brooding period combined with extended hatching periods imposes a logistical limit on the number of larvae from different parents that can be reared and maintained simultaneously. The eggs were fertilized by a male with a carapace length of 83.2 mm and were brooded for 328 days. Hatching began in late February 2013 and was extended over 24 days (2005 larvae total, 83.6 ± 183.4 larvae per day). Individuals used for respiration studies were obtained from a different female (pre-moult carapace length 73.4 mm; post-moult carapace length 81.3 mm) that moulted during the night of 19 June 2012 and oviposited the following day. The eggs were fertilized by a male with carapace length 96.4 mm and were brooded for 327 days. Hatching began in mid-May 2013 and was extended over 18 days (2903 larvae total, 161.3 ± 365.9 larvae per day). Freshly hatched larvae were collected in filters placed within the outflow of the aquarium where the gravid female was isolated. Larvae hatched predominantly at night and consequently larvae were removed each morning, ensuring larval age varied by no more than 24 h. Larvae were isolated individually within 100 ml rearing cups (temperature 6°C, 24 h darkness, atmospheric pressure). Larvae were inspected daily and water changes were made every other day. Morphological changes and the presence of exuvia were used to determine larval stage [32]. The larval development of L. maja is lecithotrophic [32] and therefore larval stages were maintained without food; the first juvenile crab stage was fed freshly hatched Artemia salina nauplii ad libitum every other day and the water was changed before feeding. The juvenile crabs were starved for 3 days prior to starting pressure treatments to avoid molecular contamination from gut contents.
(c). Pressure treatments
Acute pressure exposures were performed on individuals from the zoea I, megalopa and crab I stages, and across a range of different hydrostatic pressures: 0.1–30.0 MPa. Five individuals were used for most pressure treatments, except where numbers were limiting and only four individuals were used—these are highlighted in the results. In separate, yet comparable treatments, oxygen consumption rates (MO2, µmol O2 mg−1 h−1) were measured to assess respiratory response to acute increases in hydrostatic pressure. Oxygen consumption rates were assessed following an adaptation of established protocols [47,48]. Owing to larval mortality, fewer crab I were available for these treatments; therefore, fewer pressure treatments were carried out: 0.1, 10.0 and 15.0 MPa. Three vials without individuals were used for each pressure treatment to control for microbial respiration within the seawater.
For all treatments, individuals were placed within separate 2.8 ml cryovials filled with seawater pre-incubated to 6°C. The vials were filled until they overflowed and were carefully capped underwater to avoid trapping air within the vial. The vials were placed within a steel pressure vessel (Stauff, Germany), which was filled with fresh water [49]. Both the water and the pressure vessel were pre-incubated for 24 h at 6°C. The vessel was then pressurized to the selected pressure using a Maximator M72 manual hydraulic pump: experimental pressure was reached within 10 s [49]. The vessel was kept at constant temperature (6°C) and pressure for 4 h. For transcriptional analysis, individuals were removed from the pressure vials, transferred to 1.5 ml centrifuge tubes and flash frozen whole in liquid nitrogen, within 5 min of instantaneous depressurization. Samples were stored at −80°C until further analysis. For respiration rate analysis, vials were gently inverted three times to ensure homogeneity of seawater oxygen within the vial. The vial lid was then removed, and the oxygen saturation of the water was determined using an oxygen micro-optode connected to a Microx TX3 array (PreSens, Germany), calibrated according to manufacturer's instructions. The animal was then removed from the vial and gently blotted on tissue paper, transferred to a pre-weighed tin capsule and frozen at −80°C for subsequent biomass (dry mass) determination.
(d). Respiratory rate measurements and statistical analysis
Oxygen consumption was calculated from the difference between the mean oxygen saturation in the control vials and the oxygen saturation in the treatment vials following established methods for determining oxygen concentration in air-saturated seawater [50]. Respiration rates were normalized according to biomass (dry mass), determined by freeze drying and subsequently weighing the samples.
The respiratory responses of each ontogenetic stage to pressure treatments were evaluated independently, as fewer treatments were carried out for crab I compared with the other stages. The data were analysed using a one-way ANOVA, as they were normally distributed and homoscedastic (Shapiro–Wilk and Levene's test, respectively; p > 0.05).
(e). RNA extraction and reverse transcription
Whole individuals were transferred from −80°C storage to liquid nitrogen and then to a 5 ml tube containing 2.2 ml TRI-reagent (Sigma-Aldrich, UK), were homogenized, and total RNA was extracted following the manufacturer's protocol. Total RNA was assessed for purity and integrity using a Nanodrop spectrophotometer (Thermo Fischer Scientific) and Experion (Bio-Rad), respectively. Subsequently, total RNA was DNase-treated using Promega RQ1 RNase-free DNase (Promega, UK) following the manufacturer's protocol, and 0.68 μl of DNase-treated total RNA was reverse-transcribed in a 20 μl reaction using Superscript III (Invitrogen, UK) and oligo (dT)23 primers according to the manufacturer's protocol.
(f). Primer design
Degenerate PCR-based gene hunting techniques were used to generate L. maja-specific gene sequences for seven genes: the narg gene, CS gene, two hsp70 isoforms, and three candidate reference genes, tubulin α1, eef1a and rpl8. Reference genes are used as internal controls to correct for differences in starting cDNA template quantities in quantitative PCR (qPCR) [51]. Degenerate primer pairs were designed for each gene from alignments of sequences from crustacean and insect species available in sequence databases (GenBank, EMBL-EBI) using ClustalW v. 2.0.12 [52]. Regions of the putative target genes were amplified by PCR reactions using the degenerate primers and L. maja template cDNA. The resulting amplicons were gel extracted and cloned using the pGem-T easy vector kit (Promega, UK). Colonies with the appropriate insert were grown, plasmids were extracted and sequences were determined using conventional dideoxy-termination sequencing (Source BioScience, UK). Primers for use in qPCR were designed from the determined L. maja gene sequences (electronic supplementary material S1) using standard criteria and Primer Express software (Applied Biosystems, USA).
(g). Quantitative PCR
Quantitative PCR (qPCR) combines simultaneous amplification and fluorescent detection, enabling accurate quantification of expression levels within the samples. To ensure primer specificity and efficiency, each qPCR primer pair was optimized in order to determine the optimum primer concentrations and reaction efficiencies, ensuring 90–105% efficiency across the predicted cDNA concentration range (electronic supplementary material S2) [51,53]. A melt curve analysis was also applied to ensure that all samples produced a single distinct product.
All reactions were carried out on the Mx3005P qPCR system (Agilent, UK). Each 25 μl reaction contained 12.5 μl Precision 2× qPCR Master mix (Primer-Design, UK) containing SYBR green fluorescent dye, 1 μl (approx. 34 ng) of template cDNA and 0.5 μl ROX Reference Dye (Invitrogen, UK). Two technical replicates were run for each sample, in addition to negative controls for every gene. Standard qPCR conditions were applied (electronic supplementary material S1).
Quantification cycle (Cq) values were analysed using qBase+ software (Biogazelle, UK [38]). The Cq values for each of the genes of interest (narg, CS, hsp70a and hsp70b genes) were normalized against the best reference genes (eef1a and rpl8 genes, determined using the geNorm function within the qBase+ software [54]). These normalized relative quantities were scaled relative to the 0.1 MPa atmospheric control treatment for each gene and are therefore presented as relative fold change (RFC) values. Comparisons between 0.1, 10.0, 20.0, 25.0 and 30.0 MPa treatments within each life stage were made by one-way ANOVA in conjunction with a post hoc Tukey test.
3. Results
(a). Sequences identified by gene hunting
Six consensus sequence fragments were determined in L. maja by degenerate PCR-based gene hunting, producing significant BLAST hits. These sequences were deposited in the EMBL-EBI database. A 947 bp fragment (accession no. LN713467) was translated as a putative elongation factor 1-alpha (eef1α gene) that shared a 96% amino acid identity (query coverage = 99%, E-value = 0.0) with the crab Macrophthalmus japonicas (electronic supplementary material S3a). A 449 bp fragment (LN713464) was translated as a putative ribosomal protein L8 (rpl8 gene), and shared a 94% amino acid identity (query coverage = 99%, E-value = 4 × 10−98) with the shrimp Litopenaeus vannamei (electronic supplementary material S3b). A 1206 bp fragment (LN713465) was translated as a putative NMDAR-regulated protein 1 (NARP 1) (narg) that shared a 62% amino acid identity (query coverage = 100%, E-value = 1 × 10−179) with the termite Zootermopsis nevadensis (electronic supplementary material S3c). This amino acid sequence was also identified as containing a predicted NARP 1 domain (Pfam-A domain prediction). Two fragments, both 485 bp in length, were translated as putative 70 kDa heat-shock protein (hsp70) isoforms. The first fragment (LN713468), named hsp70a, shared a 93% amino acid identity (query coverage = 84%, E-value = 1 × 10−86) with the crab Goniopsis cruentata (electronic supplementary material S3d). The second fragment (LN713469), named hsp70b, shared an 88% amino acid identity (query coverage = 84%, E-value = 2 × 10−77) with the crab Cancer pagurus (electronic supplementary material S3e). The nucleotide and translated amino acid sequences for both putative hsp70 isoforms were aligned and showed distinctiveness (electronic supplementary material S4). Their identity as distinct isoforms of the hsp70 gene was further confirmed by expression analysis, where each isoform showed differential expression patterns (described below). Finally, a 423 bp fragment (accession no. LN713466) was translated as a putative citrate synthase protein (CS) that shared 86% amino acid identity (coverage = 99%, E-value = 8 × 10−84) with the beetle Dendroctonus ponderosae (electronic supplementary material S3f).
(b). Pressure effect on respiration rate
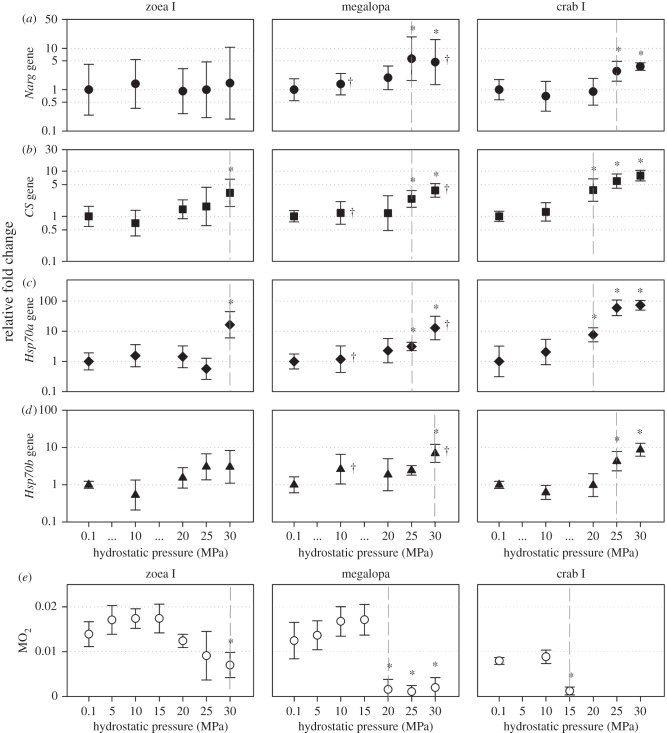
Hydrostatic pressure significantly affected respiratory rate (MO2, µmol O2 mg−1 h−1), for all ontogenetic stages (figure 1). The hydrostatic pressure at which significant decreases in oxygen consumption were first observed shifted with increasing ontogenetic age, with significant decrease at 30.0 MPa in zoea I stage (F6,28 = 8.555, p < 0.001), at 20.0 MPa in the megalopa stage (F6,28 = 31.157, p < 0.001) and at 15 MPa in the crab I stage (F2,12 = 72.431, p < 0.001).
Figure 1.
The northern stone crab Lithodes maja. Relative fold changes (RFCs) and 95% CIs of four genes. (a) The narg gene (circles); (b) the CS gene (squares); (c) the hsp70a gene (diamonds); (d) the hsp70b gene (triangles); and (e) the rate of oxygen consumption with SD (MO2, µmol O2 mg−1 h−1) across three early ontogenetic life stages at hydrostatic pressures ranging from 0.1 MPa (atmospheric control) to 30 MPa (equivalent of 3000 m water depth). RFCs scaled to the 0.1 MPa control treatment. Statistical significance is displayed as *p< 0.05, calculated from five biological replicates unless otherwise stated. †Four biological replicates. Vertical dashed lines visually emphasize the hydrostatic pressure at which significant differences are first observed.
(c). Pressure effect on relative gene expression
The effect of hydrostatic pressure on the relative transcription of all genes was dependent on ontogenetic stage (figure 1). In the zoea I stage, there were no significant RFCs of the narg gene or the hsp70b gene, but both the CS gene and hsp70a gene showed a significant fold increase at 30.0 MPa in the zoea I stage (RFC 3.3, p < 0.05; 16.4, p < 0.01). In the megalopa stage, significant differences were seen at 25.0 and 30.0 MPa in the narg gene (RFC 5.6 and 4.6, respectively, p < 0.001), the CS gene (RFC 2.4 and 3.8, respectively, p < 0.05) and the hsp70a gene (RFC 3.1 and 12.8, respectively, p < 0.01); there was also a significant fold increase of the hsp70b gene at 30.0 MPa (RFC 7.0, p < 0.01). Finally, in the crab I stage, significant fold increases were seen at 25.0 and 30.0 MPa in the narg gene (RFC 2.8 and 3.6, p < 0.01) and the hsp70b gene (RFC 4.3 and 8.7, p < 0.01), and increases at 20.0, 25.0 and 30.0 MPa in the CS gene (RFC 3.8, 6.1 and 8.0, respectively, p < 0.05) and the hsp70a gene (RFC 7.7, 59.7 and 73.1, respectively, p < 0.01).
4. Discussion
This study is the first to provide patterns in transcriptional responses to hydrostatic pressure in a continental slope-depth-adapted species, demonstrating a decrease in the acute pressure tolerance limit as ontogeny advances. This presents a significant advance in describing the potential physiological mechanisms that control bathymetric distributions. Such knowledge could be used to assess the ability of species to respond to environmental change by shifting their bathymetric distributions, and may also be useful for the reconstruction of physiological bottlenecks in the radiation history of species.
In this study, all three early ontogenetic stages of L. maja are able to tolerate exposure to pressures beyond that of their adult depth distribution (4–790 m); however, the pressure at which respiratory and transcriptional pressure effects are first observed decreases between the zoea I, megalopa and crab I stages. These results are consistent with a number of studies that have examined the effects of pressure on the embryonic and larval forms of several shallow-water benthic invertebrates. These studies reveal some general trends in ontogenetic pressure tolerance shifts: initial increases in tolerance from early embryonic to early larval forms are followed by subsequent decreases in tolerance in later life-history stages [55]. Without exception, all stages tolerated pressures greater than those found in the adult depth range [14].
Respiratory responses observed in this study support the observation of an ontogenetic shift in the upper hydrostatic pressure tolerance in L. maja. Increases in pressure above acclimation pressure typically result in an increase in oxygen consumption, followed by a subsequent decline or inhibition of oxygen consumption [11,47,56–58]. As with aerobic thermal windows, acute external increases in hydrostatic pressure beyond the ‘optimum range’ are proposed to lead to increases in homeostatic effort, and a corresponding increase in mitochondrial activity [11,58,59]. In the absence of adaptations to increased hydrostatic pressure, impaired membrane and enzyme functionality at critical upper pressures may lead to reduced metabolism, and a mismatch between oxygen demand and oxygen supply, resulting in a shift from aerobic to anaerobic metabolism [11,57,59,60]. While tolerable in the short term, long-term survival in this state is not possible [61]. This change in mitochondrial activity in response to hydrostatic pressure may be reflected in the expression of the citrate synthase (CS) gene.
Acute exposure to elevated hydrostatic pressure also affected transcription of two known members of the CSR, 70 kDa heat-shock protein isoforms hsp70a and hsp70b [62]. These results are consistent with previous studies demonstrating increased transcription of genes coding for heat-shock proteins in response to the effects of high hydrostatic pressure and/or low temperature [26,27,29]. Increases in hydrostatic pressure have long been known to affect protein folding, impacting quaternary, tertiary and secondary structure [63]. The upregulation of two HSP70 isoforms provides additional evidence for macromolecular damage in L. maja in response to elevated hydrostatic pressure.
Further evidence of cellular damage may be inferred by the upregulation of the narg gene, putatively coding for NMDA receptor-regulated protein 1. It is proposed that when approaching critical pressure limits, neuronal cell death leads to an upregulation of genes associated with NMDA receptor activity [29]. Significant upregulation of the narg gene in response to increases in hydrostatic pressure in the temperate shallow-water shrimp Palaemonetes varians may be an indicator for the onset of pressure intolerances associated with neuronal tissue sensitivity [29]. This upregulation is proposed to be a precursor to behavioural signs of pressure intolerance such as ‘loss of equilibrium’ (where the shrimp are observed lying motionless on their side or dorsal surface for more than 2 s) [29,57]. However, the sequence of transcriptional and respiratory responses identified in L. maja in all stages (significant decrease in respiration rate prior to narg transcriptional response) contrasts with the sequence in the shallow-water shrimp P. varians at acclimation temperature, where the narg transcriptional response in stage 3 juveniles precedes the significant decrease in respiration rate observed in adult shrimp (cf. [29,57]). Although this contrast suggests functional nervous pressure adaptation in L. maja, the evolutionary and ecological distances between these taxa prevent definitive conclusion. Regardless, this study supports the hypothesis that hydrostatic pressure affects critical biological processes, and potentially imposes a lower bathymetric limit beyond which adaptations are required [11,26,27,29,57,58,64–66].
Aerobic thermal tolerance windows have been shown to change throughout ontogeny in fish. Tolerance windows initially increase from the early embryonic stages to the juvenile stage, as diffusion across the cell surface is replaced by a ventilation and circulatory system [67]. Subsequently, thermal tolerance windows narrow with increasing age and metabolic demands [67]. Theories of thermal tolerance are often extended to hydrostatic pressure tolerance, because the effects of high pressure and low temperature are thought to be analogous [11,64]. A narrowing of pressure tolerance through ontogeny in L. maja may be associated with a number of morphological and functional changes. Many crustacean larvae experience a shift in cardiac function through early development. For example, in the shrimp Metapenaeus ensis and isopod Ligia oceanica, a transition from myogenic to neurogenic cardiac control has been observed [68,69]. In the king crab L. santolla, the cardiac region becomes delineated from the megalopa to the juvenile [70], which may coincide with a shift in cardiac function [71]. The gastric region also becomes delineated from the megalopa to the juvenile in L. santolla [70], reflecting the transition from endotrophy to exotrophy, which is associated with an increase in the activities of digestive enzymes [72]. In L. maja, there are significant differences in biomass, oxygen consumption and chemical composition between the zoea, megalopa and juvenile stages [32].
The three deep-sea lithodid genera have distinct generalized lower depth limits: Lithodes has the shallowest distribution (approx. 200–1250 m, 2.1–12.6 MPa), Paralomis occupies intermediate depths (approx. 200–2000 m, 2.1–20.2 MPa) and Neolithodes has the deepest distribution (approx. 2000–3500 m, 20.2–35.2 MPa) [31]. In this study, all three life stages of L. maja were able to tolerate acute pressure conditions beyond the equivalent pressures of the known adult distribution (4–790 m, approx. 0.14–8.0 MPa). Respiration rate measurements for the crab I stage indicate that oxygen consumption is significantly reduced at 15 MPa (equivalent to approx. 1500 m), the first of the examined pressures to fall beyond the maximum observed depth of the genus Lithodes (approx. 1250 m, 12.6 MPa) [31]. While these data are an important first step towards fully understanding the role of pressure tolerance in the ecology and evolution of marine taxa, it is not possible to fully determine the role of pressure limitation in L. maja without knowledge of adult tolerance limits, and without a sustained pressure treatment to assess the long-term effects of pressure and potential acclimation. To date, such long-term studies are completely lacking from the literature, mostly because of the lack of adequate technology. Available studies have largely focused on acute pressure exposures ranging from seconds to few hours, and rarely extend to more than a few days (reviewed in [14]). This study provides an important bridge for longer-term exposure studies in adults, and provides an indication of the short-term role of hydrostatic pressure limitation in early ontogenetic stages.
Hydrostatic pressure tolerance may affect ontogenetic migration patterns in lithodid crabs. While little is known about the ecology of the early ontogeny of L. maja, there is evidence of ontogenetic migration in Lithodes ferox. There is almost no overlap between the bathymetric distribution of adults and juveniles, and juvenile L. ferox are almost exclusively found in deeper waters than adults [73]. Although the sub-Antarctic king crab L. santolla displays the opposite bathymetric distribution, this pattern may relate to the specific behavioural ecology of this species: juveniles form dense aggregations around shallow kelp holdfasts at depths of 3–5 m [74]. In Paralithodes camtschaticus, seasonal migration into shallow waters has been observed and is related to larval release, a migratory process likely to be driven by planktotrophic larval development [75].
In summary, we demonstrate a shift in the short-term, acute high-pressure tolerance limit between the zoea I, megalopa and crab I stages of L. maja as evidenced from changes in gene expression and aerobic metabolism. While further studies on the effects of pressure at every level of biological organization, and through the full life cycle, are necessary to understand better the mechanisms underlying these physiological limits, this study provides the first indication of the molecular response to hydrostatic pressure in the early ontogeny of a continental slope-depth-adapted species, and indicates that bathymetric distribution may become more constrained as ontogeny advances.
Supplementary Material
Acknowledgements
We thank staff at the Sven Lovén Centre for Marine Sciences—Kristineberg, Sweden, for supporting this work; special thanks go to Bengt Lundve for organizing the sampling of crabs and their maintenance prior to transportation, and to Tony Roysson for leading the fieldwork. We thank Adam Reed for detailed advice on aquarium construction, which enabled successful long-term maintenance of adult crabs at NOCS.
Data accessibility
DNA sequences: EMBL-EBI/GenBank accessions LN713464 to LN713469. Sequence alignments, qPCR assay efficiencies, and primer sequences for each gene listed in this article have been uploaded as part of electronic supplementary material. Clustal Omega alignment files are available from the Dryad data repository system (http://dx.doi.org/10.5061/dryad.d6t40).
Authors' contributions
J.P.M., S.T., A.B. and C.H. conceived the work; C.M. and A.B. reared the larvae; C.M. and J.P.M. carried out the molecular laboratory work; A.B. carried out respiratory rate measurements; C.M. drafted the manuscript; all authors contributed to manuscript writing.
Competing interests
We declare we have no competing interests.
Funding
This study was supported by a grant from ASSEMBLE (FP7) to S.T. C.M. was supported by the Society for Underwater Technology's educational support fund. J.P.M. and A.B. were supported by PhD studentships from the National Environment Research Council (NERC).
References
- 1.Jablonski D, Sepkoski JJ, Bottjer DJ, Sheehan PM. 1983. Onshore-offshore patterns in the evolution of Phanerozoic shelf communities. Science 222, 1123–1125. ( 10.1126/science.222.4628.1123) [DOI] [PubMed] [Google Scholar]
- 2.Hall S, Thatje S. 2009. Global bottlenecks in the distribution of marine Crustacea: temperature constraints in the family Lithodidae. J. Biogeogr. 36, 2125–2135. ( 10.1111/j.1365-2699.2009.02153.x) [DOI] [Google Scholar]
- 3.Lindner A, Cairns SD, Cunningham CW. 2008. From offshore to onshore: multiple origins of shallow-water corals from deep-sea ancestors. PLoS ONE 3, e2429 ( 10.1371/journal.pone.0002429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horne DJ. 1999. Ocean circulation modes of the Phanerozoic: implications for the antiquity of deep-sea benthonic invertebrates. Crustaceana 72, 999–1018. ( 10.1163/156854099503906) [DOI] [Google Scholar]
- 5.McClain CR, Hardy SM. 2010. The dynamics of biogeographic ranges in the deep sea. Proc. R. Soc. B 277, 3533–3546. ( 10.1098/rspb.2010.1057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rogers AD. 2000. The role of the oceanic oxygen minima in generating biodiversity in the deep sea. Deep-Sea Res. II 47, 119–148. ( 10.1016/S0967-0645(99)00107-1) [DOI] [Google Scholar]
- 7.Jacobs DK, Lindberg DR. 1998. Oxygen and evolutionary patterns in the sea: onshore/offshore trends and recent recruitment of deep-sea faunas. Proc. Natl Acad. Sci. USA 95, 9396–9401. ( 10.1073/pnas.95.16.9396) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raupach MJ, Mayer C, Malyutina M, Wägele J-W. 2009. Multiple origins of deep-sea Asellota (Crustacea: Isopoda) from shallow waters revealed by molecular data. Proc. R. Soc. B 276, 799–808. ( 10.1098/rspb.2008.1063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson GD. 1999. Some of the deep-sea fauna is ancient. Crustaceana 72, 1019–1030. ( 10.1163/156854099503915) [DOI] [Google Scholar]
- 10.Carney RS. 2005. Zonation of deep biota on continental margins. Oceanogr. Mar. Biol. 43, 211–278. [Google Scholar]
- 11.Brown A, Thatje S. 2011. Respiratory response of the deep-sea amphipod Stephonyx biscayensis indicates bathymetric range limitation by temperature and hydrostatic pressure. PLoS ONE 6, e28562 ( 10.1371/journal.pone.0028562) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Etter R, Rex MA, Chase MR, Quattro JM. 2005. Population differentiation decreases with depth in deep-sea bivalves. Evolution 59, 1479–1491. ( 10.1111/j.0014-3820.2005.tb01797.x) [DOI] [PubMed] [Google Scholar]
- 13.Etter RJ, Boyle EE, Glazier A, Jennings RM, Dutra E, Chase MR. 2011. Phylogeography of a pan-Atlantic abyssal protobranch bivalve: implications for evolution in the Deep Atlantic. Mol. Ecol. 20, 829–843. ( 10.1111/j.1365-294X.2010.04978.x) [DOI] [PubMed] [Google Scholar]
- 14.Brown A, Thatje S. 2014. Explaining bathymetric diversity patterns in marine benthic invertebrates and demersal fishes: physiological contributions to adaptation of life at depth. Biol. Rev. 89, 406–426. ( 10.1111/brv.12061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rex MA, Etter RJ. 2010. Deep-sea biodiversity: pattern and scale. Cambridge, MA: Harvard University Press. [Google Scholar]
- 16.Pradillon F, Gaill F. 2007. Pressure and life: some biological strategies. Rev. Environ. Sci. Biotechnol. 6, 181–195. ( 10.1007/s11157-006-9111-2) [DOI] [Google Scholar]
- 17.Cossins AR, Macdonald AG. 1989. The adaptation of biological membranes to temperature and pressure: fish from the deep and cold. J. Bioenerg. Biomembr. 21, 115–135. ( 10.1007/BF00762215) [DOI] [PubMed] [Google Scholar]
- 18.Hazel JR. 1995. Thermal adaptation in biological membranes: is homeoviscous adaptation the explanation? Annu. Rev. Physiol. 57, 19–42. ( 10.1146/annurev.ph.57.030195.000315) [DOI] [PubMed] [Google Scholar]
- 19.Somero GN. 1995. Proteins and temperature. Annu. Rev. Physiol. 57, 43–68. ( 10.1146/annurev.ph.57.030195.000355) [DOI] [PubMed] [Google Scholar]
- 20.Hazel JR, Williams E. 1990. The role of alterations in membrane lipid composition in enabling physiological adaptation of organisms to their physical environment. Prog. Lipid Res. 29, 167–227. ( 10.1016/0163-7827(90)90002-3) [DOI] [PubMed] [Google Scholar]
- 21.Siebenaller JF, Garrett DJ. 2002. The effects of the deep-sea environment on transmembrane signaling. Comp. Biochem. Physiol. B 131, 675–694. ( 10.1016/S1096-4959(02)00027-1) [DOI] [PubMed] [Google Scholar]
- 22.Kültz D. 2005. Molecular and evolutionary basis of the cellular stress response. Annu. Rev. Physiol. 67, 225–257. ( 10.1146/annurev.physiol.67.040403.103635) [DOI] [PubMed] [Google Scholar]
- 23.Barshis DJ, Ladner JT, Oliver TA, Seneca FO, Traylor-Knowles N, Palumbi SR. 2013. Genomic basis for coral resilience to climate change. Proc. Natl Acad. Sci. USA 110, 1387–1392. ( 10.1073/pnas.1210224110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evans TG, Hofmann GE. 2012. Defining the limits of physiological plasticity: how gene expression can assess and predict the consequences of ocean change. Phil. Trans. R. Soc. B 367, 1733–1745. ( 10.1098/rstb.2012.0019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pespeni MH, et al. 2013. Evolutionary change during experimental ocean acidification. Proc. Natl Acad. Sci. USA 110, 6937–6942. ( 10.1073/pnas.1220673110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cottin D, Brown A, Oliphant A, Mestre NC, Ravaux J, Shillito B, Thatje S. 2012. Sustained hydrostatic pressure tolerance of the shallow water shrimp Palaemonetes varians at different temperatures: Insights into the colonisation of the deep sea. Comp. Biochem. Physiol. A 162, 357–363. ( 10.1016/j.cbpa.2012.04.005) [DOI] [PubMed] [Google Scholar]
- 27.Cottin D, Shillito B, Chertemps T, Thatje S, Léger N, Ravaux J. 2010. Comparison of heat-shock responses between the hydrothermal vent shrimp Rimicaris exoculata and the related coastal shrimp Palaemonetes varians. J. Exp. Mar. Biol. Ecol. 393, 9–16. ( 10.1016/j.jembe.2010.06.008) [DOI] [Google Scholar]
- 28.Ravaux J, Léger N, Rabet N, Morini M, Zbinden M, Thatje S, Shillito B. 2012. Adaptation to thermally variable environments: capacity for acclimation of thermal limit and heat shock response in the shrimp Palaemonetes varians. J. Comp. Physiol. B 182, 899–907. ( 10.1007/s00360-012-0666-7) [DOI] [PubMed] [Google Scholar]
- 29.Morris JP, Thatje S, Ravaux J, Shillito B, Fernando D, Hauton C. 2015. Acute combined pressure and temperature exposures on a shallow-water crustacean: novel insights into the stress response and high pressure neurological syndrome. Comp. Biochem. Physiol. A 181, 9–17. ( 10.1016/j.cbpa.2014.10.028) [DOI] [PubMed] [Google Scholar]
- 30.Cunningham C, Blackstone N, Buss L. 1992. Evolution of king crabs from hermit crab ancestors. Nature 355, 539–542. ( 10.1038/355539a0) [DOI] [PubMed] [Google Scholar]
- 31.Snow SM. 2010. The evolutionary history and phylogeny of the Lithodinae (Decapoda: Anomura: Lithodidae). PhD thesis, University of Southampton, School of Ocean and Earth Sciences. [Google Scholar]
- 32.Anger K. 1996. Physiological and biochemical changes during lecithotrophic larval development and early juvenile growth in the northern stone crab, Lithodes maja (Decapoda: Anomura). Mar. Biol. 126, 283–296. ( 10.1007/BF00347453) [DOI] [Google Scholar]
- 33.Anger K. 2001. The biology of decapod crustacean larvae. Rotterdam, The Netherlands: AA Balkema Publishers. [Google Scholar]
- 34.Thatje S. 2004. Reproductive trade-offs in benthic decapod crustaceans of high southern latitudes: tolerance of cold and food limitation. Rep. Polar Mar. Res. 483, 1–183. [Google Scholar]
- 35.Thatje S, Anger K, Calcagno JA, Lovrich GA, Pörtner H-O, Arntz WE. 2005. Challenging the cold: crabs reconquer the Antarctic. Ecology 86, 619–625. ( 10.1890/04-0620) [DOI] [Google Scholar]
- 36.Sørensen JG, Kristensen TN, Loeschcke V. 2003. The evolutionary and ecological role of heat shock proteins. Ecol. Lett. 6, 1025–1037. ( 10.1046/j.1461-0248.2003.00528.x) [DOI] [Google Scholar]
- 37.Lemos D, Salomon M, Gomes V, Phan VN, Buchholz F. 2003. Citrate synthase and pyruvate kinase activities during early life stages of the shrimp Farfantepenaeus paulensis (Crustacea, Decapoda, Penaeidae): effects of development and temperature. Comp. Biochem. Physiol. B 135, 707–719. ( 10.1016/S1096-4959(03)00166-0) [DOI] [PubMed] [Google Scholar]
- 38.Seibel BA, Childress JJ. 2000. Metabolism of benthic octopods (Cephalopoda) as a function of habitat depth and oxygen concentration. Deep-Sea Res. I 47, 1247–1260. ( 10.1016/S0967-0637(99)00103-X) [DOI] [Google Scholar]
- 39.Seibel BA, Drazen JC. 2007. The rate of metabolism in marine animals: environmental constraints, ecological demands and energetic opportunities. Phil. Trans. R. Soc. B 362, 2061–2078. ( 10.1098/rstb.2007.2101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seibel BA, Thuesen EV, Childress JJ. 2000. Light-limitation on predator-prey interactions: consequences for metabolism and locomotion of deep-sea cephalopods. Biol. Bull. 198, 284–298. ( 10.2307/1542531) [DOI] [PubMed] [Google Scholar]
- 41.Thuesen EV, McCullough KD, Childress JJ. 2005. Metabolic enzyme activities in swimming muscle of medusae: is the scaling of glycolytic activity related to oxygen availability? J. Mar. Biol. Assoc. UK 85, 603–611. ( 10.1017/S0025315405011537) [DOI] [Google Scholar]
- 42.Brauer RW, Török Z. 1984. Hydrostatic pressure effects on the central nervous system: perspectives and outlook [and discussion]. Phil. Trans. R. Soc. Lond. B 304, 17–30. ( 10.2307/2396150) [DOI] [PubMed] [Google Scholar]
- 43.Mor A, Grossman Y. 2006. Modulation of isolated N-methyl-d-aspartate receptor response under hyperbaric conditions. Eur. J. Neurosci. 24, 3453–3462. ( 10.1111/j.1460-9568.2006.05233.x) [DOI] [PubMed] [Google Scholar]
- 44.Mor A, Grossman Y. 2007. High pressure modulation of NMDA receptor dependent excitability. Eur. J. Neurosci. 25, 2045–2052. ( 10.1111/j.1460-9568.2007.05479.x) [DOI] [PubMed] [Google Scholar]
- 45.Millan MH, Wardley-Smith B, Halsey MJ, Meldrum BS. 1989. Studies on the role of the NMDA receptor in the substantia nigra pars reticulata and entopeduncular nucleus in the development of the high pressure neurological syndrome in rats. Exp. Brain Res. 78, 174–178. ( 10.1007/BF00230696) [DOI] [PubMed] [Google Scholar]
- 46.Mor A, Kuttner YY, Levy S, Merav M, Hollmann M, Grossman Y. 2012. Pressure-selective modulation of NMDA receptor subtypes may reflect 3D structural differences. Front. Cell. Neurosci. 6, 37 ( 10.3389/fncel.2012.00037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith KE, Thatje S. 2012. The secret to successful deep-sea invasion: does low temperature hold the key? PLoS ONE 7, e51219 ( 10.1371/journal.pone.0051219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thatje S, Casburn L, Calcagno JA. 2010. Behavioural and respiratory response of the shallow-water hermit crab Pagurus cuanensis to hydrostatic pressure and temperature. J. Exp. Mar. Biol. Ecol. 390, 22–30. ( 10.1016/j.jembe.2010.04.028) [DOI] [Google Scholar]
- 49.Mestre NC, Thatje S, Tyler PA. 2009. The ocean is not deep enough: pressure tolerances during early ontogeny of the blue mussel Mytilus edulis. Proc. R. Soc. B 276, 717–726. ( 10.1098/rspb.2008.1376) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benson BB, Krause D., Jr 1984. The concentration and isotopic fractionation of oxygen dissolved in freshwater and seawater in equilibrium with the atmosphere. Limnol. Oceanogr. 29, 620–632. ( 10.4319/lo.1984.29.3.0620) [DOI] [Google Scholar]
- 51.Bustin SA, Beaulieu J-F, Huggett J, Jaggi R, Kibenge FS, Olsvik PA, Penning LC, Toegel S. 2010. MIQE precis: practical implementation of minimum standard guidelines for fluorescence-based quantitative real-time PCR experiments. BMC Mol. Biol. 11, 74 ( 10.1186/1471-2199-11-74) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Larkin MA, et al. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23, 2947–2948. ( 10.1093/bioinformatics/btm404) [DOI] [PubMed] [Google Scholar]
- 53.Bustin SA, et al. 2009. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55, 611–622. ( 10.1373/clinchem.2008.112797) [DOI] [PubMed] [Google Scholar]
- 54.Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J. 2007. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 8, R19 ( 10.1186/gb-2007-8-2-r19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mestre NC, Brown A, Thatje S. 2013. Temperature and pressure tolerance of larvae of Crepidula fornicata suggest thermal limitation of bathymetric range. Mar. Biol. 160, 743–750. ( 10.1007/s00227-012-2128-x) [DOI] [Google Scholar]
- 56.Mickel TJ, Childress J. 1982. Effects of pressure and pressure acclimation on activity and oxygen consumption in the bathypelagic mysid Gnathophausia ingens. Deep Sea Res. A 29, 1293–1301. ( 10.1016/0198-0149(82)90009-7) [DOI] [Google Scholar]
- 57.Oliphant A, Thatje S, Brown A, Morini M, Ravaux J, Shillito B. 2011. Pressure tolerance of the shallow-water caridean shrimp Palaemonetes varians across its thermal tolerance window. J. Exp. Biol. 214, 1109–1117. ( 10.1242/jeb.048058) [DOI] [PubMed] [Google Scholar]
- 58.Thatje S, Robinson N. 2011. Specific dynamic action affects the hydrostatic pressure tolerance of the shallow-water spider crab Maja brachydactyla. Naturwissenschaften 98, 299–313. ( 10.1007/s00114-011-0768-1) [DOI] [PubMed] [Google Scholar]
- 59.Frederich M, Pörtner HO. 2000. Oxygen limitation of thermal tolerance defined by cardiac and ventilatory performance in spider crab, Maja squinado. Am. J. Physiol. 279, R1531–R1538. [DOI] [PubMed] [Google Scholar]
- 60.Weiss M, Heilmayer O, Brey T, Lucassen M, Pörtner H-O. 2012. Physiological capacity of Cancer setosus larvae—adaptation to El Niño Southern Oscillation conditions. J. Exp. Mar. Biol. Ecol. 413, 100–105. ( 10.1016/j.jembe.2011.11.023) [DOI] [Google Scholar]
- 61.Pörtner H-O. 2002. Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp. Biochem. Physiol. A 132, 739–761. ( 10.1016/S1095-6433(02)00045-4) [DOI] [PubMed] [Google Scholar]
- 62.Feder ME, Hofmann GE. 1999. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu. Rev. Physiol. 61, 243–282. ( 10.1146/annurev.physiol.61.1.243) [DOI] [PubMed] [Google Scholar]
- 63.Silva J, Weber G. 1993. Pressure stability of proteins. Annu. Rev. Phys. Chem. 44, 89–113. ( 10.1146/annurev.pc.44.100193.000513) [DOI] [PubMed] [Google Scholar]
- 64.Airriess CN, Childress JJ. 1994. Homeoviscous properties implicated by the interactive effects of pressure and temperature on the hydrothermal vent crab Bythograea thermydron. Biol. Bull. 187, 208–214. ( 10.2307/1542243) [DOI] [PubMed] [Google Scholar]
- 65.Brown A, Thatje S. 2015. The effects of changing climate on faunal depth distributions determine winners and losers. Glob. Change Biol. 21, 173–180. ( 10.1111/gcb.12680) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Somero GN. 1992. Adaptations to high hydrostatic pressure. Annu. Rev. Physiol. 54, 557–577. ( 10.1146/annurev.ph.54.030192.003013) [DOI] [PubMed] [Google Scholar]
- 67.Pörtner HO, Farrell AP. 2008. Physiology and climate change. Science 322, 690–692. ( 10.1126/science.1163156) [DOI] [PubMed] [Google Scholar]
- 68.McMahon B, Tanaka K, Doyle J, Chu K. 2002. A change of heart: cardiovascular development in the shrimp Metapenaeus ensis. Comp. Biochem. Physiol. A 133, 577–587. ( 10.1016/S1095-6433(02)00196-4) [DOI] [PubMed] [Google Scholar]
- 69.Yamagishi H, Hirose E. 1997. Transfer of the heart pacemaker during juvenile development in the isopod crustacean Ligia exotica. J. Exp. Biol. 200, 2393–2404. [DOI] [PubMed] [Google Scholar]
- 70.McLaughlin PA, Anger K, Kaffenberger A, Lovrich GA. 2001. Megalopal and early juvenile development in Lithodes santolla (Molina, 1782) (Decapoda: Anomura: Paguroidea: Lithodidae), with notes on zoeal variations. Invertebr. Reprod. Dev. 40, 53–67. ( 10.1080/07924259.2001.9652498) [DOI] [Google Scholar]
- 71.Spicer JI. 2001. Development of cardiac function in crustaceans: patterns and processes. Am. Zool. 41, 1068–1077. ( 10.1093/icb/41.5.1068) [DOI] [Google Scholar]
- 72.Saborowski R, Thatje S, Calcagno J, Lovrich G, Anger K. 2006. Digestive enzymes in the ontogenetic stages of the southern king crab, Lithodes santolla. Mar. Biol. 149, 865–873. ( 10.1007/s00227-005-0240-x) [DOI] [Google Scholar]
- 73.Abelló P, Macpherson E. 1991. Distribution patterns and migration of Lithodes ferox (Filhol) (Anomura: Lithodidae) off Namibia. J. Crustacean Biol. 11, 261–268. ( 10.2307/1548363) [DOI] [Google Scholar]
- 74.Cárdenas CA, Cañete JI, Oyarzún S, Mansilla A. 2007. Podding of juvenile king crab Lithodes santolla (Molina, 1782) (Crustacea) in association with holdfasts of Macrocystis pyrifera (Linnaeus) C. Agardh, 1820. Invest. Mar. 35, 105–110. ( 10.4067/S0717-71782007000100010) [DOI] [Google Scholar]
- 75.Jørgensen LL, Nilssen EM. 2011. The invasive history, impact and management of the red king crab Paralithodes camtschaticus off the coast of Norway. In In the wrong place—alien marine crustaceans: distribution, biology and impacts (eds Galil BS, Clark PF, Carlton JT.), pp. 521–536. Dordrecht, The Netherlands: Springer. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
DNA sequences: EMBL-EBI/GenBank accessions LN713464 to LN713469. Sequence alignments, qPCR assay efficiencies, and primer sequences for each gene listed in this article have been uploaded as part of electronic supplementary material. Clustal Omega alignment files are available from the Dryad data repository system (http://dx.doi.org/10.5061/dryad.d6t40).