Figure 4.
KCa3.1 Sets Node of Ranvier Membrane Potential and Preserves Nodal Excitability
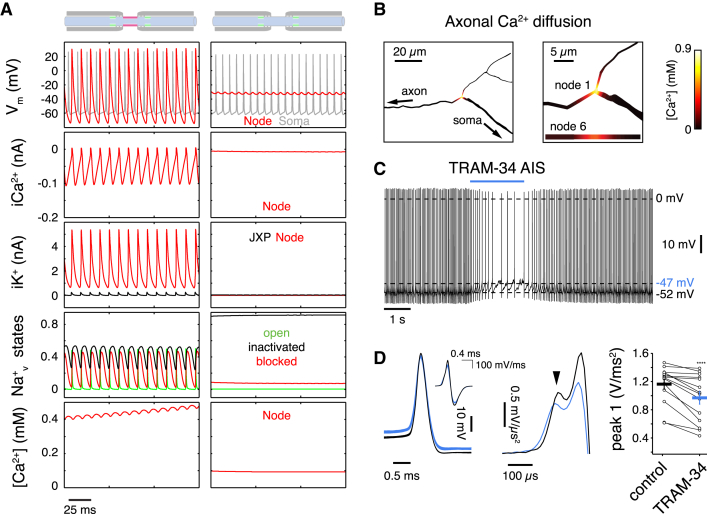
(A) Data obtained from a PC multicompartmental model. NoR (red, sixth node, 1,820 μm from soma) and somatic (gray) APs (Vm, top row) during spontaneous firing in a Purkinje cell model with and without NoR KCa3.1 channels. Left column: AP propagation is sustained by nodal gKCa3.1 (0.05 S/cm2) and juxtaparanodal (JP) delayed rectifier K+ channels (0.002 S/cm2). Right column: lack of axonal KCa3.1 causes depolarization block at the NoR. Second row: Ca2+ current at the NoR. Third row: total K+ current at the JP and NoR, respectively, for each model. Fourth row: fractional resurgent Nav states during spontaneous firing of each respective model. Bottom row: local Ca2+ concentration.
(B) Activity-dependent axonal Ca2+ diffusion in the model axon.
(C) Somatic recording from a spontaneously firing Purkinje cell during local application of 1 μM TRAM-34 to the AIS. TRAM-34 causes a depolarizing shift in Vm and a reduction of firing rate.
(D) Example APs (left) before (black) and during TRAM-34 (blue) application illustrate TRAM-34-induced change in Vm. Average second derivative of somatic APs (middle). Arrowhead indicates first peak originating in the axon. Summary data show a TRAM-induced reduction in the amplitude of the first axonal peak of the second derivative of the somatic action potential (right). Horizontal bars indicate average ±SEM (n = 15), p < 0.0005.