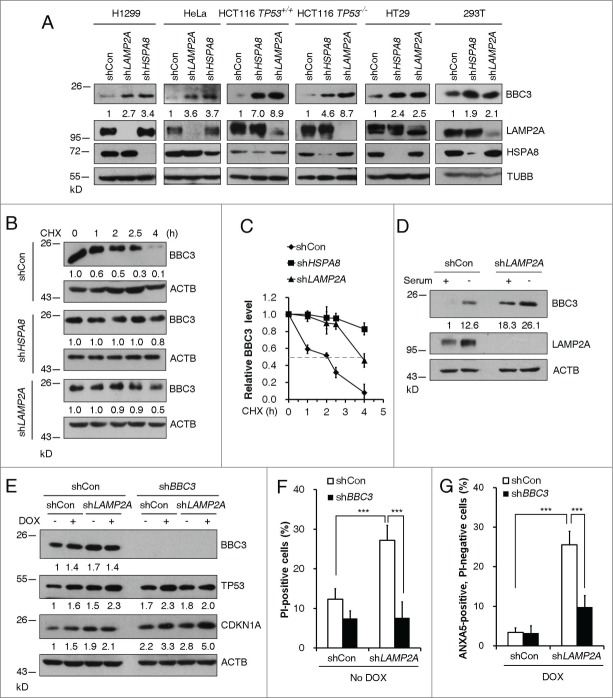
Figure 1.
Inhibition of CMA leads to BBC3 induction. (A) Representative western blots and densitometric data (n = 3 or 4) showing that CMA ablation leads to BBC3 upregulation. The indicated tumor cells were infected with vectors containing control, LAMP2A, or HSPA8 shRNA and then lysed. (B) Representative immunoblots (of n ≥ 3) showing that loss of CMA stabilizes BBC3. H1299 cells expressing the indicated shRNAs were treated with 40 μg/ml CHX and then harvested at the indicated times. (C) Relative BBC3 protein level shown in (B) was quantified. (D) Representative Western blots (n = 3) showing that LAMP2A depletion further promotes BBC3 induction upon serum withdrawal. H1299 cells were infected with control or LAMP2A shRNA lentiviruses for 48 h followed by serum deprivation for 48 h. Cell lysates were harvested for immunoblotting analysis. (E) Western blotting analysis of BBC3, TP53, and CDKN1A levels in HCT116 cells. Cells first infected with vectors containing shCon or BBC3 shRNAs, then with shCon or LAMP2A shRNAs were treated with 0.5 μM DOX for 6 h and then harvested. (F) FACS analysis of cell death by PI staining in HCT116 cells stably expressing shRNA constructs as in (E) showing that CMA inhibition induces cell death in a BBC3-dependent manner. (G) Percentage of early apoptotic cells after 24 h of DOX exposure in cells stably expressing shRNA constructs as in (E) was determined by ANXA5 and PI staining, which shows CMA inhibition induces cell death upon DNA damage in a BBC3-dependent manner. Data were represented as mean ±SEM; P < 0.01** and 0.001***, n = 4, t test. Quantification of BBC3 protein levels was done relative to loading control.