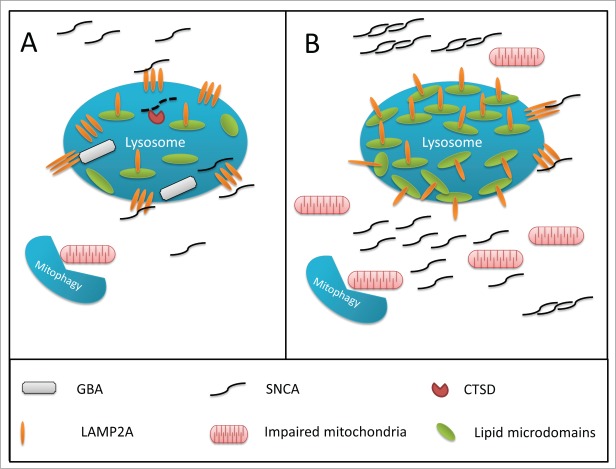
Figure 2.
Hypothesized mechanism for GBA-associated lysosomal dysfunction in Parkinson disease. (A) In a normally functioning lysosome, GBA is associated with the inner part of the lysosomal membrane, and degrades glucocerebrosides to glucose and ceramide, thus controlling the proper composition of the membrane. The chaperone-mediated autophagy receptor, LAMP2A, is able to freely move out of lipid rafts, create complexes, and internalize SNCA into the lysosome for degradation. (B) Impaired GBA activity can affect the composition of the membrane, leading to an increased density of lipid rafts on the lysosomal membrane. In this scenario, it is more difficult for LAMP2A to create the complexes required for the internalization of SNCA into the lysosome, leading to SNCA accumulation. This effect of GBA impairment on the lysosomal membrane may interrupt other pathways, and affect macroautophagy and mitophagy, which will lead to the accumulation of damaged mitochondria and increased oxidative stress.