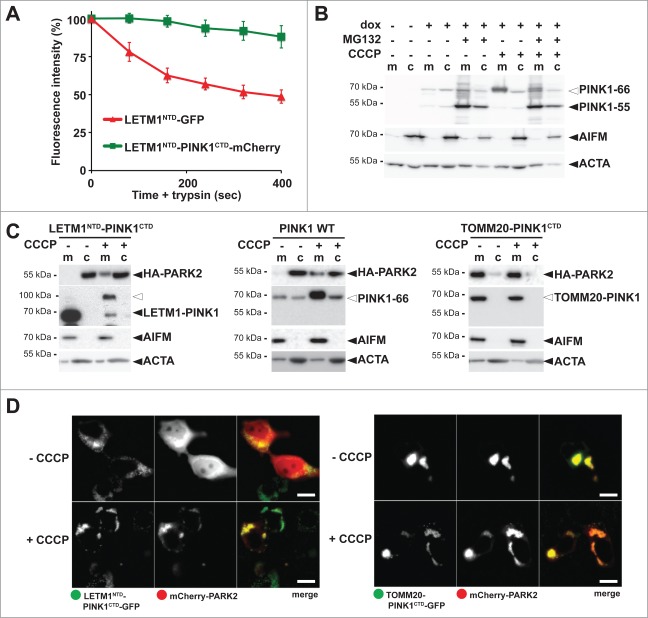
Figure 4.
PINK1 stably anchored to the inner membrane cannot recruit PARK2. (A) The C terminus comprising the PINK1 kinase domain delays mitochondrial import as shown by quantitative FPP assay of HEK293T cells coexpressing LETM1NTD-GFP and LETM1NTD-PINK1CTD-mCherry (>60 cells were analyzed, n = 3, means ±SEM ). See Figure 3A for experimental outline and Figure S4B for representative picture. (B) PARL-catalyzed cleavage of ectopically expressed PINK1–66 in HEK293 T-REx cells led to proteasomal degradation of PINK1–55 as shown by sensitivity to MG132 (2 µM) compared to vehicle control. (C) PINK1 anchored in the inner membrane (LETM1NTD-PINK1CTD) interacted with PARK2 only in CCCP-treated cells, whereas the outer membrane targeted TOMM20-PINK1CTD chimera recruited PARK2 independently of uncoupling of the mitochondrial membrane potential. Cells were fractionized and recruitment of ectopically expressed HA-PARK2 from the soluble nonmitochondrial fraction (c) to mitochondria (m) was analyzed. The cellular markers AIFM and ACTA/actin were used as fractionation control. White triangle indicates unprocessed pre form of LETM1-PINK1 observed upon block of mitochondrial import (D) Whereas expression of LETM1NTD-PINK1CTD-GFP did not lead to recruitment of mCherry-PARK2 to mitochondria, coexpression of TOMM20-PINK1CTD-GFP was sufficient to stabilize PARK2 on the mitochondrial surface even in absence of dissipation of the membrane potential by CCCP. Scale bars: 10 μm.