Figure 7.
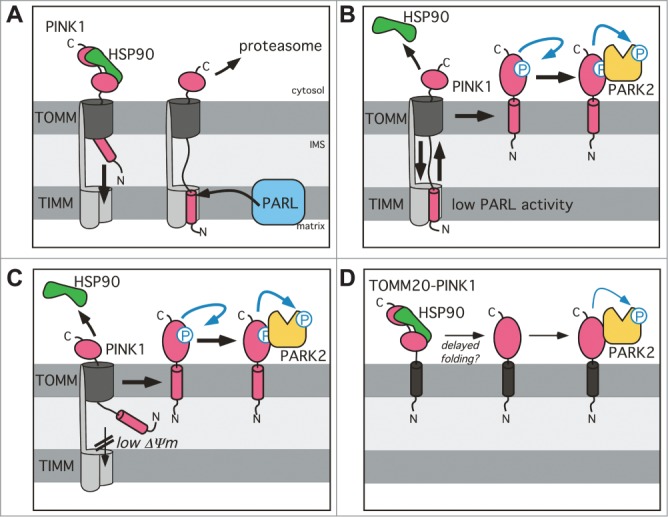
Model for the PARL-PINK1-PARK2 relay in mitochondrial quality control. (A) Due to prolonged interaction with the cytosolic chaperone HSP90, PINK1–66 forms an import intermediate spanning the IMS and the TOMM complex. PARL-catalyzed cleavage of the PINK1 TM domain in the inner mitochondrial membrane generates soluble PINK1–55, which due to lack of its membrane anchor slides back into the cytosol, where it becomes rapidly degraded by the proteasome. (B) Unprocessed PINK1–66, observed upon PARL ablation, stays competent for sliding back to the outer membrane where it is subsequently integrated into the lipid bilayer by an unknown mechanism. Outer membrane targeting is accompanied by PINK1 autophosphorylation, PARK2 recruitment and its PINK1-dependent phosphorylation. (C) Upon loss of the inner mitochondrial membrane potential (Δψ Upon lo–66 accumulates in the outer membrane similar to the situation in PARL-ablated cells. Although integration of the TM domain into the inner membrane is blocked, we postulate that the PINK1CTD transiently inserts into the TOMM channel leading to stripping of cytosolic HSP90 and subsequent efficient maturation of outer membrane targeted PINK1–66. (D) Direct targeting of PINK1CTD to the outer membrane by fusion to TOMM20 (black) is sufficient to trigger PARK2 recruitment, but lack of autophosphorylation suggests that due prolonged interaction with cytosolic factors such as HSP90 folding of the kinase domain is hampered probably leading to reduced activation.
