Abstract
A series of 3-[2′-(Substitutedbenzylideneamino)phenyl]-2-methyl-6-substituted quinazolin-4-ones (5–10), 3-[2′-(3″-chloro-2″-oxo-4″-substitutedphenylazetidin-1″-yl)phenyl]-2-methyl-6-substitutedquinazolin-4-ones (11–16), and 3-[2′-(2″-substitutedphenyl-4″-oxo-1″,3″-thiazolidin-3″-yl)phentl]-2-methyl-6-substitutedquinazolin-4-ones (17–22) have been synthesized in the present study. The structures of the synthesized compounds were assigned on the basis of elemental analysis, IR, 1H NMR, and mass spectral data. All the newly synthesized compounds were screened for anti-inflammatory and analgesic activities.
1. Introduction
Quinazolinone derivatives represent one of the most active classes of compounds possessing a wide spectrum of biological activity. They are widely used in pharmaceuticals and agrochemicals. Several reports have been published on the biological activities of quinazolinone derivatives, including their anti-inflammatory [1–7], antimalarial [8, 9], antimicrobial, anti-fungal, antibacterial [10–16], anticonvulsant [17–20], and antitumor [21, 22] activities. Moreover, large number of quinazolinone derivatives having substitution at 2 and 3 position by different heterocyclic moieties increases anti-inflammatory potential of quinazolinone derivatives. Similarly, various azetidinones [23–25] and thiazolidinones [26, 27] have been reported to possess potent anti-inflammatory activity. Looking to the medicinal importance of 4(3H)-quinazolinone, 4-thiazolidinone, and azetidinones, we report here the synthesis of a new class of heterocyclic molecules in which all of these moieties are present and try to develop potential bioactive molecules. The structures of the compounds synthesized were assigned on the basis of elemental analysis, IR, 1H NMR, and Mass spectral data. These compounds were evaluated for their anti-inflammatory and analgesic activities.
2. Materials and Methods
2.1. Chemistry
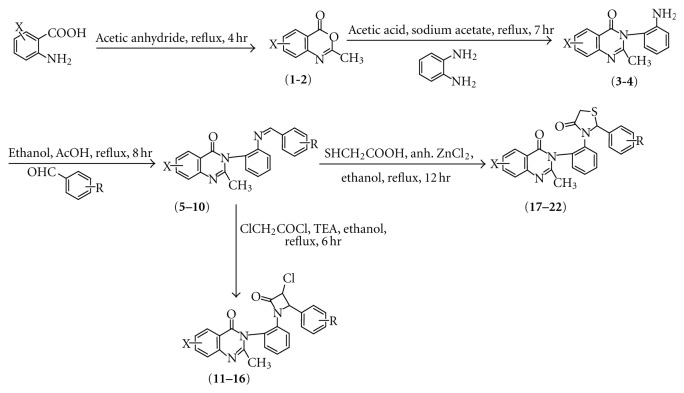
The synthetic routes of compounds are outlined in Scheme 1. As shown in Scheme 1, compounds 2-methylsubstitued-4H-3,1-benzoxazin-4-ones (1,2) were synthesized by the known procedure of Bogert [28]. Substituted anthranilic acid reacted with acetic anhydride to give 2-methylsubstituted-4H-3,1-benzoxazin-4-ones (1,2), which further reacted with 2-amino phenyl amine in acetic acid to yield 3-(2′-aminophenyl)-2-methyl-6-substituted quinazolin-4-ones (3,4). Compounds (3,4) when treated with different substituted aromatic aldehydes formed various substituted arylidene derivatives (5–10). These arylidene derivatives on treatment with chloroacetylchloride in presence of triethylamine yielded 3-[2′-(3′′-chloro-2′′-oxo-4′′-substituted phenylazetidin-1′′-yl)phenyl]-2-methyl-6-substitutedquinazolin-4-ones (11–16). On the other hand, these arylidene derivatives on reaction with thioglycolic acid and anhydrous ZnCl2 furnished 3-[2′-(2′′-substitutedphenyl-4′′-oxo-1′′,3′′-thiazolidin-3′′-yl)phenyl]-2-methyl-6-substitutedquinazolin-4-ones. (17–22). The structures of the compounds synthesized were assigned on the basis of elemental analysis, IR, 1H NMR, and Mass spectral data.
Scheme 1.
2.2. Methods
2.2.1. Biological Activity
The experiments were performed with albino rats of the Charles-foster stain of either sex, excluding pregnant females, of 70–95 d. weighting 100–150 g. Food (chaw pallet) and water were given to the animals ad libitum. All the compounds were dissolved in propylene glycol. Phenylbutazone was used as reference drug.
Anti-Inflammatory Activity against Carrageenan-Induced Rat's Paw Oedema
This study was done with the procedure of Winter et al. [29]. The rats were divided into three groups (control, treated, and standard drug) of six animals each. A freshly prepared suspension of carrageenan (1% in 0.9% saline) 0.05 mn was injected under the planter aponeurosis of the right hind paw of each rat. Test compounds and standard drug were administered orally to the animals of drug-treated groups and the standard drug group, respectively, 1 h before the carrageenan injection. The paw volume of each rat was measured before 1 and after 3 h of carrageenan treatment with the help of a plethysmometer. The percent anti-inflammatory activity was calculated according to the formula given below
| (1) |
where V t and V c are the mean increase in paw volume of rats of the treated and the control group, respectively. Results were statistically analyzed.
Analgesic Activity
This activity was assessed following the method of Berkowitz et al. [30]. This method is based on the property of the test compound to antagonize the phenyl quinone-induced pain syndrome in mice. Groups of five mice were injected intraperitoneally with 0.25 mL of a 0.02% solution of phenylquinone in ethanol (5%) 1 h after of oral administration of the test compound. The number of writhes induced in each mouse was counted for 5 min (between 5 and 10 min) after injection of an irritant. The analgesic effect was expressed as percent protection in comparison to control.
| (2) |
2.2.2. Experimental
Melting points were determined in one-end-open capillary tube on Thomas Hoover melting apparatus and are uncorrected. The purities of compounds were checked by thin layer chromatography (TLC) using silica gel-G coated plats. The spots were developed in an iodine chamber and visualized under ultraviolet (UV) lamp. Infrared (IR) absorption spectra were recorded on Perkin-Elmer RX-1 FTIR spectrometer using potassium bromide (KBr) pellet, and wave numbers were given in cm−1. The 1H NMR spectra were recorded in CDCl3 on a Bruker Avance II 400 NMR spectrometer (400 MHz). The 13C NMR spectra were recorded in CDCl3 on a Bruker Avance II 400 NMR spectrometer operating at 100 MHz. The chemical shifts are reported in part per million (δ ppm) using trimethylsilane (TMS) as an internal standard. Elemental analysis (C, H, N) was performed on Perkin-Elmer 2400 analyzer, and values were either in ±0.4% of the calculated.
3-(2′-Aminophenyl)-2-Methyl-Quinazolin-4-one ( 3)
A mixture of 2-methyl-4H-3,1-benzoxazin-4-one (1) (0.01 mole) with 2-aminophenylamine (0.01 mole) in acetic acid (40 mL) was refluxed for 7 hr. in the presence of sodium acetate. The solvent was removed under reduced pressure. The solid thus obtained was filtered off and recrystallized from toluene to give compound 3 (78%). m.p. 99°C. IR (KBr) ν max in cm−1: 3033 (CH aromatic), 2920 (CH aliphatic), 1690 (C=O), 1595 (C=N); 1HNMR (CDCl3) δ in ppm: 9.10 (s, 2H, NH2 exchangeable), 6.89–7.61 (m, 8H, Ar–H), 2.29 (s, 3H, CH3); Anal. Calcd. for C15H13N3O: C, 71.71; H, 5.18; N, 16.73: Found: C, 71.85; H, 5.20; N, 16.68: MS [M]+ at m/z 251.
The following compound was prepared using a similar procedure described for compound 3.
3-(2′-Aminophenyl)-2-Methyl-6-Bromoquinazolin-4-one ( 4)
(75%) (Benzene) m.p. 199°C, IR (KBr) ν max in cm−1: 3030 (CH aromatic), 2925 (CH aliphatic), 1695 (C=O), 1598 (C=N); 1H NMR (CDCl3) δ in ppm: 9.06 (s, 2H, NH2 exchangeable), 6.85–7.51 (m, 7H, Ar–H), 2.24 (s, 3H, CH3); Anal. Calcd. for C15H12N3OBr: C, 54.55; H, 3.64; N, 12.73: Found: C, 54.38; H, 3.65; N, 12.76: MS [M]+ at m/z 330.
3-[ 2′-(Benzylideneamino)Phenyl]-2-Methyl-Quinazolin-4-one ( 5)
A solution of compound 3 (0.01 moles) in ethanol (55 mL) was refluxed with benzaldehyde (0.01 moles) separately in the presence of glacial acetic acid (2.5 mL) for 11 hr. The reaction mixture was concentrated, cooled, and then poured into ice water. The separated solid was filtered and recrystallized from methanol to give compound 29 (63%) m.p. 118°C: IR (KBr) ν max in cm−1: 3028 (CH aromatic), 2930CH aliphatic), 1705 (C=O), 1610 (C=N); 1HNMR (CDCl3) δ in ppm: 6.79–7.63 (m, 13H, Ar–H), 6.35 (s, 1H, N=CH), 2.21 (s, 3H, CH3); Anal. Caled. for C22H17N3O: C, 77.88; H, 5.01; N, 12.39: Found: C, 77.96; H, 5.00; N, 12.41: MS [M]+ at m/z. 339.
The following compounds were prepared using a similar procedure described for compound 5.
3-[2′-(p-Chlorobenzylideneamino)Phenyl]-2-Methyl-Quinazolin-4-one ( 6)
(65%). (Benzene) m.p. 134°C: IR (KBr) ν max in cm−1: 3028 (CH aromatic), 2929 (CH aliphatic), 1705 (C=O), 1598 (C=N) 788 (C–Cl); 1H NMR (CDCl3) δ in ppm: 6.82–7.65 (m, 12H, Ar–H), 6.38 (s, 1H, N=CH), 2.24 (s, 3H, CH3); Anal. Caled. for C22H16N3OCl: C,70.68; H, 4.28; N, 11.24: Found: C, 70.79; H, 4.27; N, 11.26: MS [M]+ at m/z. 373.5.
3-[2′-(p-Methoxybenzylideneamino)Phenyl]-2-Methyl-Quinazolin-4-one ( 7)
(60%). (Methanol) m.p. 148°C: IR (KBr) ν max in cm−1: 3035(CH aromatic), 2932 (CH aliphatic), 1700 (C=O), 1600 (C=N); 1HNMR (CDCl3) δ in ppm: 6.80–7.61 (m, 12H, Ar–H), 6.40 (s, 1H, N=CH), 3.48 (s, 3H, OCH3) 2.26 (s, 3H, CH3); Anal. Caled. for C23H19N3O2: C, 74.80; H, 5.15; N, 11.38: Found: C, 74.96; H, 5.17; N, 11.29: MS [M]+ at m/z. 369.
3-[ 2′-(Benzylideneamino)Phenyl]-2-Methyl-6-Bromoquinazolin-4-one ( 8)
(64%). (Ethanol) m.p. 215°C: IR (KBr) ν max in cm−1: 3033 (CH aromatic), 2926 (CH aliphatic), 1698 (C=O), 1606 (C=N); 1H NMR (CDCl3) δ in ppm: 6.83–7.60 (m, 12H, Ar–H), 6.43 (s, 1H, N=CH), 2.22 (s, 3H, CH3); Anal. Caled. for C22H16N3OBr: C,63.16; H, 3.83; N, 10.05: Found: C, 63.29; H, 3.82; N, 10.07: MS [M]+ at m/z. 418.
3-[ 2′-(p-Chlorobenzylideneamino)Phenyl]-2-Methyl-6-Bromoquinazolin-4-one ( 9)
(65%). (Ethanol) m.p. 234°C: IR (KBr) ν max in cm−1: 3030 (CH aromatic), 2930 (CH aliphatic), 1695 (C=O), 1601 (C=N), 785 (C–Cl); 1HNMR (CDCl3) δ in ppm: 6.81–7.59 (m, 11H, Ar–H), 6.41 (s, 1H, N=CH), 2.25 (s, 3H, CH3); Anal. Caled. for C22H15N3OBrCl: C, 58.34; H, 3.31; N, 9.28: Found: C, 58.55; H, 3.32; N, 9.30: MS [M]+ at m/z. 452.5.
3-[2′-(p-Methoxybenzylideneamino)Phenyl]-2-Methyl-6-Bromoquinazolin-4-one ( 10)
(60%). (Acetone) m.p. 223°C: IR (KBr) ν max in cm−1: 3033 (CH aromatic), 2926 (CH aliphatic), 1698 (C=O), 1595 (C=N); 1HNMR (CDCl3) δ in ppm: 6.83–7.61 (m, 11H, Ar–H), 6.44 (s, 1H, N=CH), 3.44 (s, 3H, OCH3) 2.25 (s, 3H, CH3); Anal. Caled. for C23H18N3O2Br: C, 61.61; H, 4.02; N, 9.38: Found: C, 61.52; H, 4.01; N, 9.36: MS [M]+ at m/z. 448
3-[2′-(3′′-Chloro- 2′′-Oxo- 4′′-Phenylazetidin- 1′′-Yl)-Phenyl]-2-Methyl-Quinazol-In-4-one ( 11)
To a solution of compound 5 (0.01 mole) and triethylamine (0.5 mL) in ethanol (55 mL) was added in monochloroacetylchloride (0.014 mole) at 50°C. The reaction mixture was stirred for 45 min. at room temperature and refluxed for 8 hr. The reaction mixture was filtered to removed triethylamine hydrogen chloride and the resultant solution was poured onto crushed ice with constant stirring. The solid thus obtained was recrystallized from acetone to give desired compound 11 (60%) m.p. 153°C. IR (KBr): ν max in cm−1: IR (KBr) ν max in cm−1: 3030 (CH aromatic), 2925 (CH aliphatic), 1700 (C=O), 1595 (C=N); 1HNMR (CDCl3) δ in ppm: 6.81–7.98 (m, 13H, Ar–H), 6.51 (d,1H, J = 9.0 Hz, CH–Cl), 4.60 (d, 1H, J = 9.3 Hz, N–CH–Ar), 2.21 (s, 3H, CH3);. Anal. Calcd. for C24H18N3O2Cl: C, 69.31; H, 4.33; N, 10.11: Found: C, 69.47; H, 4.32; N, 10.14: MS [M]+at m/z 415.5.
The following compounds were prepared using a similar procedure described for compound 11.
3-[2′-(3′′-Chloro- 2′′-Oxo- 4′′-(p-Chlorophenyl)Azetidin- 1′′-yl)-Phenyl]-2-Methyl Quinazolin-4-one ( 12)
(58%) (Methanol) m.p. 198°C. IR (KBr): ν max in cm−1: IR (KBr) ν max in cm−1: 3033 (CH aromatic), 2928 (CH aliphatic), 1705 (C=O), 1591 (C=N), 788 (C–Cl); 1HNMR (CDCl3) δ in ppm: 6.83–7.95 (m, 12H, Ar–H), 6.56 (d,1H, J = 8.8 Hz, CH–Cl), 4.62 (d, 1H, J = 9.0 Hz, N–CH–Ar), 2.25 (s, 3H, CH3);. Anal. Calcd. for C24H17N3O2Cl2: C, 64.00; H, 3.78; N, 9.33: Found: C, 64.15; H, 3.80; N, 9.30: MS [M]+at m/z 450.
3-[2′-(3′′-Chloro- 2′′-Oxo- 4′′-(p-Methoxyphenyl)Azetidin- 1′′-yl)-Phenyl]-2-Methyl Quinazolin-4-one ( 13)
(56%) (Ethanol)m.p. 212°C. IR (KBr): ν max in cm−1: IR (KBr) ν max in cm−1 : 3028 (CH aromatic), 2930 (CH aliphatic), 1700 (C=O), 1595 (C=N); 1HNMR (CDCl3) δ in ppm: 6.81–7.93 (m, 12H, Ar–H), 6.54 (d,1H, J = 9.0 Hz, CH–Cl), 4.65 (d, 1H, J = 9.3 Hz, N–CH–Ar), 3.47 (s, 3H, OCH3) 2.23 (s, 3H, CH3);. Anal. Calcd. for C25H20N3O3Cl: C, 67.34; H, 4.49; N, 9.43: Found: C, 67.15; H, 4.50; N, 9.40: MS [M]+at m/z 445.5.
3-[ 2′-(3′′-Chloro-2′′-Oxo-4′′-Phenylazetidin-1′′-yl)-Phenyl]-2-Methyl-6-Bromo-Quinazolin-4-one ( 14)
(58%) (Methanol) m.p. 176°C. IR (KBr): ν max in cm−1: IR (KBr) ν max in cm−1 : 3030 (CH aromatic), 2925 (CH aliphatic), 1695 (C=O), 1595 (C=N); 1HNMR (CDCl3) δ in ppm: 6.80–7.92 (m, 12H, Ar–H), 6.54 (d, 1H, J = 9.2 Hz, CH–Cl), 4.64 (d, 1H, J = 9.1 Hz, N–CH–Ar), 2.22 (s, 3H, CH3);. Anal. Calcd. for C24H17N3O2ClBr: C, 58.24; H, 3.44; N, 8.49: Found: C, 58.36; H, 3.43; N, 8.51: MS [M]+at m/z 494.5.
3-[ 2′-(3′′-Chloro-2′′-Oxo-4′′-(p-Chlorophenyl)Azetidin-1′′-yl)-Phenyl]-2-Methyl-6-Bromo Quinazolin-4-one ( 15)
(54%) (Ethanol) m.p. 204°C. IR (KBr): ν max in cm−1: IR (KBr) ν max in cm−1: 3027 (CH aromatic), 2930 (CH aliphatic), 1710 (C=O), 1598 (C=N), 785 (C–Cl); 1HNMR (CDCl3) δ in ppm: 6.79–7.90 (m, 11H, Ar–H), 6.55 (d, 1H, J = 9.3 Hz, CH–Cl), 4.61 (d, 1H, J = 8.8 Hz, N–CH–Ar), 2.20 (s, 3H, CH3);. Anal. Calcd. for C24H16N3O2Cl2Br: C, 54.44; H, 3.02; N, 7.94: Found: C, 54.61; H, 3.01; N, 7.95: MS [M]+at m/z 529.
3-[ 2′-(3′′-Chloro-2′′-Oxo-4′′-(P-Methoxyphenyl)Azetidin-1′′-yl)-Phenyl]-2-Methyl Quinazolin-4-one ( 16)
(60%) (Acetone) m.p. 246°C. IR (KBr): ν max in cm−1: IR (KBr) ν max in cm−1: 3030 (CH aromatic), 2930 (CH aliphatic), 1700 (C=O), 1590 (C=N); 1HNMR (CDCl3) δ in ppm: 6.80–7.92 (m, 11H, Ar–H), 6.52 (d, 1H, J = 9.2 Hz, CH–Cl), 4.62 (d, 1H, J = 9.0 Hz, N–CH–Ar), 3.49 (s, 3H, OCH3) 2.25 (s, 3H, CH3);. Anal. Calcd. for C25H19N3O3ClBr: C, 57.20; H, 3.62; N, 8.01: Found: C, 57.31; H, 3.61; N, 8.06: MS [M]+at m/z 524.5.
3-[ 2′-(2′′-Phenyl-4′′-Oxo-1′′,3′′-Thiazolidin-3′′-yl)Phenyl]-2-Methyl-6-Quinazolin-4-one ( 17)
A mixture of compound 5(0.01 moles), thioglycolic acid (0.012 moles) in absolute dioxane (75 mL) and in the presence of anhydrous ZnCl2 was refluxed for 18 hr. The solvent was removed under reduced pressure. The solid thus obtained was filtered and washed with water. The product finally obtained was dried and recrystallized from ethanol to yield compound 17 (55%) m.p. 164°C. IR (KBr): ν max in cm−1: IR (KBr) ν max in cm−1 : 3032 (CH aromatic), 2931 (CH aliphatic), 1705 (C=O), 1595 (C=N),685 (C–S–C); 1HNMR (CDCl3) δ in ppm: 6.80–7.97 (m, 13H, Ar–H), 4.55 (s, 1H, N–CH–Ar), 4.25 (s, 2H, CH2) 2.25 (s, 3H, CH3);. Anal. Calcd. for C24H19N3O2S: C, 69.73; H, 4.60; N, 10.17: Found: C, 69.82; H, 4.61; N, 10.22: MS [M]+at m/z 413.
3-[ 2′-(2′′-(p-Chlorophenyl)-4′′-Oxo-1′′,3′′-Thiazolidin-3′′-Yl)Phenyl]-2-Methyl-Quinazolin-4-one. ( 18)
(49%) (Methanol) m.p. 192°C. IR (KBr): ν max in cm−1: IR (KBr) ν max in cm−1 : 3030 (CH aromatic), 2930 (CH aliphatic), 1700 (C=O), 1598 (C=N), 784 (C–Cl), 688 (C–S–C); 1HNMR (CDCl3) δ in ppm: 6.83–7.96 (m, 12H, Ar–H), 4.58 (s, 1H, N–CH–Ar), 4.21 (s, 2H, CH2) 2.22 (s, 3H, CH3);. Anal. Calcd. for C24H18N3O2SCl: C, 64.36; H, 4.02; N, 9.39: Found: C,64.41; H, 4.03; N, 9.35: MS [M]+at m/z 447.5.
3-[2′-(2′′-(p-Methoxyphenyl)- 4′′-Oxo- 1′′, 3′′-Thiazolidin- 3′′-yl)Phenyl]-2-Methyl Quinazolin-4-one ( 19)
(50%) (Acetone) m.p. 204°C. IR (KBr): ν max in cm−1: IR (KBr) ν max in cm−1 : 3032 (CH aromatic), 2927 (CH aliphatic), 1700 (C=O), 1600 (C=N), 690 (C–S–C),; 1HNMR (CDCl3) δ in ppm: 6.81–7.95 (m, 12H, Ar–H), 4.52 (s, 1H, N–CHvAr), 4.23 (s, 2H, CH2) 3.51 (s, 3H, OCH3), 2.20 (s, 3H, CH3);. Anal. Calcd. for C25H21N3O3S: C, 67.72; H, 4.74; N, 9.48: Found: C,67.88; H, 4.73; N, 9.51: MS [M]+at m/z 443.
3-[ 2′-(2′′-Phenyl-4′′-Oxo-1′′,3′′-Thiazolidin-3′′-Yl)Phenyl]-2-Methyl-6-Bromo Quinazolin-4-one ( 20)
(55%) (Ethanol) m.p. 236°C. IR (KBr): ν max in cm−1: IR (KBr) ν max in cm−1: 3028 (CH aromatic), 2933 (CH aliphatic), 1705 (C=O), 1600 (C=N), 690 (C–S–C); 1H NMR (CDCl3) δ in ppm: 6.81–7.94 (m, 12H, Ar–H), 4.56 (s, 1H, N–CH–Ar), 4.22 (s, 2H, CH2) 2.24 (s, 3H, CH3). Anal. Calcd. for C24H18N3O2SBr: C, 58.54; H, 3.66; N, 8.54: Found: C,58.66; H, 3.67; N, 8.56: MS [M]+at m/z 492.5.
3-[2′-(2′′-(p-Chlorophenyl)- 4′′-Oxo-1′′, 3′′-Thiazolidin- 3′′-yl)Phenyl]-2-Methyl-6-Bromo Quinazolin-4-one ( 21)
(48%) (Methanol) m.p. 267°C. IR (KBr): ν max in cm−1: IR (KBr) ν max in cm−1: 3033 (CH aromatic), 2925 (CH aliphatic), 1705 (C=O), 1595 (C=N), 784 (C–Cl), 685 (C–S–C); 1HNMR (CDCl3) δ in ppm: 6.80–7.93 (m, 11H, Ar–H), 4.52 (s, 1H, N–CH–Ar), 4.24 (s, 2H, CH2) 2.26 (s, 3H, CH3);. Anal. Calcd. for C24H17N3O2SClBr: C,54.70; H, 3.23; N, 7.98: Found: C, 54.86; H, 3.22; N, 7.97: MS [M]+at m/z 526.5.
3-[2′-(2′′-(p-Methoxyphenyl)-4′′-Oxo-1′′,3′′-Thiazolidin-3′′-yl)Phenyl]-2-Methyl-6-Bromo Quinazolin-4-one ( 22)
(55%) (Ethanol) m.p. 254°C. IR (KBr): ν max in cm−1: IR (KBr) ν max in cm−1: 3031 (CH aromatic), 2928 (CH aliphatic), 1710 (C=O), 1605 (C=N), 685 (C–S–C); 1HNMR (CDCl3) δ in ppm: 6.81–7.92 (m, 11H, Ar–H), 4.59 (s,1H, N–CH–Ar), 4.25 (s, 2H, CH2), 3.49 (s, 3H, OCH3), 2.26 (s, 3H, CH3);. Anal. Calcd. for C25H20N3O3SBr: C, 57.47; H, 3.83; N, 8.05: Found: C, 57.62; H, 3.82; N, 8.03: MS [M]+at m/z 522.
3. Results and Discussion
3.1. Anti-Inflammatory Activity
The anti-inflammatory activity of newly synthesis compounds is showed in Table 1. All the compounds (5–22) were screened for anti-inflammatory activity at 50 mg/kg p.o. using phenylbutazone as reference drug. All the tested compounds exhibited 15.1 to 32.5% edema inhibition at the dose of 50 mg/kg p.o. When the compounds were substituted with p-chlorophenyl group showed better anti-inflammatory activity than phenyl group. Compounds (5–10) showed anti-inflammatory from 15.1 to 20.4%. Compound 9, which was substituted by 2′-(p-chlorobenzylideneamino)phenyl group at the IIIrd position of quinazolinone moiety showed better anti-inflammatory activity (20.4%) than compounds 5, 6, 7, 8, and 10. Cyclizations of these compounds into azetidinones and thiazolidinones have shown better anti-inflammatory activity than corresponding arylidene derivatives. Azetidinones (11–16) and thiazolidinones (17–22) have shown the anti-inflammatory activity from (24.6–27.3%) and (22.9–32.5%) respectively. In azetidinones, compound 15 showed better anti-inflammatory activity at the dose of 50 mg/kg p.o. than compounds 11, 12, 13, 14, and 16. Compound 11 exhibited lower percentage inhibition of oedema (24.6%) among azetidinones. Thiazolidinones (17–22) generally showed better anti-inflammatory then corresponding azetidinones (11–16). Among all the tested compounds, 3-[2′-(2′′-(p-chlorophenyl)-4′′-oxo-1′′,3′′-thiazolidin-3′′-yl)phenyl]-2-methyl-6-bromo quinazolin-4-one (21) showed better anti-inflammatory activity (32.5%).
Table 1.
Anti-inflammatory and analgesic data of compounds 5–22 .
| Comp. number | Dose (mg/kg p.o.) |
Anti-inflammatory activity % oedema inhibition | Analgesic activity % |
|---|---|---|---|
| 5 | 50 | 15.1 | 11.6 |
| 6 | 50 | 21.8 | 16.2 |
| 7 | 50 | 16.7 | 14.9 |
| 8 | 50 | 18.2 | 17.1 |
| 9 | 50 | 24.1 | 19.3 |
| 10 | 50 | 20.4 | 18.5 |
| 11 | 50 | 18.3 | 14.6 |
| 12 | 50 | 24.6 | 18.7 |
| 13 | 50 | 20.8 | 16.2 |
| 14 | 50 | 22.4 | 20.6 |
| 15 | 50 | 27.3 | 25.8 |
| 16 | 50 | 26.1 | 22.2 |
| 17 | 50 | 22.9 | 16.3 |
| 18 | 50 | 26.3 | 19.8 |
| 19 | 50 | 24.5 | 22.4 |
| 20 | 50 | 27.1 | 25.1 |
| 21 | 50 | 32.5 | 29.6 |
| 22 | 50 | 29.8 | 28.3 |
| Phenylbutazone | 50 | 38.8 | 36.5 |
3.2. Analgesic Activity
Compounds (5–10) have shown moderate analgesic activity (11.6–19.3%). The compound 9 showed good analgesic activity (19.3%) then compounds 5, 6, 7, 8, and 10. The formation of azetidinones (11–16) and thiazolidinones (17–22) from compounds (5–10) showed better analgesic activity than corresponding arylidene derivatives. Among these, compound 21 showed the better analgesic activity (29.6%) than other tested compounds. The results of the biological study are depicted in Table 1.
4. Conclusion
It may be concluded that the compounds having 4-chlorophenyl group of quinazolinone moiety showed better anti-inflammatory activity than phenyl moiety. Azetidinone and thiazolidinone derivatives were found active than corresponding schiff base derivatives. Further, thiazolidinones showed better anti-inflammatory and analgesics activities in comparison to their corresponding azetidinones. Among all the tested compounds, compound 21 showed better anti-inflammatory (32.5%) and analgesics (29.6%) activities than other compounds of series.
Acknowledgment
This paper is dedicated to late Professor Ashok Kumar.
References
- 1.Kumar A., Sharma S., Archana, et al. Some new 2,3,6-trisubstituted quinazolinones as potent anti-inflammatory, analgesic and COX-II inhibitors. Bioorganic and Medicinal Chemistry. 2003;11(23):5293–5299. doi: 10.1016/S0968-0896(03)00501-7. [DOI] [PubMed] [Google Scholar]
- 2.Maggio B., Daidone G., Raffa D., et al. Synthesis and pharmacological study of ethyl 1-methyl-5-(substituted 3,4-dihydro-4-oxoquinazolin-3-yl)-1H-pyrazole-4-acetates. European Journal of Medicinal Chemistry. 2001;36(9):737–742. doi: 10.1016/S0223-5234(01)01259-4. [DOI] [PubMed] [Google Scholar]
- 3.Giri R. S., Thaker H. M., Giordano T., et al. Design, synthesis and characterization of novel 2-(2,4-disubstituted-thiazole-5-yl)-3-aryl-3H-quinazoline-4-one derivatives as inhibitors of NF-κB and AP-1 mediated transcription activation and as potential anti-inflammatory agents. European Journal of Medicinal Chemistry. 2009;44(5):2184–2189. doi: 10.1016/j.ejmech.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 4.Manivannan E., Chaturvedi S. C. Analogue-based design, synthesis and molecular docking analysis of 2,3-diaryl quinazolinones as non-ulcerogenic anti-inflammatory agents. Bioorganic and Medicinal Chemistry. 2011;19(15):4520–4528. doi: 10.1016/j.bmc.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 5.Kumar A., Rajput C. S., Bhati S. K. Synthesis of 3-[4′-(p-chlorophenyl)-thiazol-2′-yl]-2-[(substituted azetidinone/thiazolidinone)-aminomethyl]-6-bromoquinazolin-4-ones as anti-inflammatory agent. Bioorganic and Medicinal Chemistry. 2007;15(8):3089–3096. doi: 10.1016/j.bmc.2007.01.042. [DOI] [PubMed] [Google Scholar]
- 6.Giri R. S., Thaker H. M., Giordano T., et al. Design, synthesis and evaluation of novel 2-thiophen-5-yl-3H-quinazolin-4-one analogues as inhibitors of transcription factors NF-κB and AP-1 mediated transcriptional activation: their possible utilization as anti-inflammatory and anti-cancer agents. Bioorganic and Medicinal Chemistry. 2010;18(7):2796–2808. doi: 10.1016/j.bmc.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Bansal E., Srivastava V. K., Kumar A. Synthesis and anti-inflammatory activity of 1-acetyl-5-substitute daryl-3-(β-aminonaphthyl)-2-pyrazolines and β-(substitute daminoethyl) amidonaphthalenes. European Journal of Medicinal Chemistry. 2001;36(1):81–92. doi: 10.1016/S0223-5234(00)01179-X. [DOI] [PubMed] [Google Scholar]
- 8.Zhu S., Wang J., Chandrashekar G., Smith E., Liu X., Zhang Y. Synthesis and evaluation of 4-quinazolinone compounds as potential antimalarial agents. European Journal of Medicinal Chemistry. 2010;45(9):3864–3869. doi: 10.1016/j.ejmech.2010.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu S., Zhang Q., Gudise C., Wei L., Smith E., Zeng Y. Synthesis and biological evaluation of febrifugine analogues as potential antimalarial agents. Bioorganic and Medicinal Chemistry. 2009;17(13):4496–4502. doi: 10.1016/j.bmc.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suresha G. P., Suhas R., Kapfo W., Channe Gowda D. Urea/thiourea derivatives of quinazolinone-lysine conjugates: synthesis and structure-activity relationships of a new series of antimicrobials. European Journal of Medicinal Chemistry. 2011;46(6):2530–2540. doi: 10.1016/j.ejmech.2011.03.041. [DOI] [PubMed] [Google Scholar]
- 11.Mohameda M. S., Kamel M. M., Kassem E. M., Abotaleb N., AbdEl-Moez S. I., Ahmed M. F. Novel 6,8-dibromo-4(3H)quinazolinone derivatives of anti-bacterial and anti-fungalactivities. European Journal of Medicinal Chemistry. 2010;45(8):3311–3319. doi: 10.1016/j.ejmech.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Patel D. R., Patel K. C. Synthesis, antimicrobial activity and application of some novel quinazolinone based monoazo reactive dyes on various fibres. Dyes and Pigments. 2011;90(1):1–10. doi: 10.1016/j.dyepig.2010.10.013. [DOI] [Google Scholar]
- 13.Kohli D., Hashim S. R., Vishal S., Sharma M., Simgh A. K. Synthesis and antibacterial activity of quinazolinone derivatives. International Journal of Pharmacy and Pharmaceutical Sciences. 2009;1(1):163–169. [Google Scholar]
- 14.Patel N. B., Patel J. C. Synthesis and antimicrobial activity of Schiff bases and 2-azetidinones derived from quinazolin-4(3H)-one. Arabian Journal of Chemistry. 2011;4(4):403–411. doi: 10.1016/j.arabjc.2010.07.005. [DOI] [Google Scholar]
- 15.Pandeya S. N., Sriram D., Nath G., De Clercq E. Synthesis, antibacterial, antifungal and anti-HIV evaluation of Schiff and Mannich bases of isatin derivatives with 3-amino-2-methylmercapto quinazolin-4(3H)-one. Pharmaceutica Acta Helvetiae. 1999;74(1):11–17. doi: 10.1016/S0031-6865(99)00010-2. [DOI] [PubMed] [Google Scholar]
- 16.Kumar A., Sharma P., Kumari P., Lal Kalal B. Exploration of antimicrobial and antioxidant potential of newly synthesized 2,3-disubstituted quinazoline-4(3H)-ones. Bioorganic and Medicinal Chemistry Letters. 2011;21(14):4353–4357. doi: 10.1016/j.bmcl.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 17.Zappalà M., Grasso S., Micale N., et al. 1-Aryl-6,7-methylenedioxy-3H-quinazolin-4-ones as anticonvulsant agents. Bioorganic and Medicinal Chemistry Letters. 2003;13(24):4427–4430. doi: 10.1016/j.bmcl.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 18.Jatav V., Mishra P., Kashaw S., Stables J. P. CNS depressant and anticonvulsant activities of some novel 3-[5-substituted 1,3,4-thiadiazole-2-yl]-2-styryl quinazoline-4(3H)-ones. European Journal of Medicinal Chemistry. 2008;43(9):1945–1954. doi: 10.1016/j.ejmech.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 19.El-Azab A. S., ElTahir K. E. H. Synthesis and anticonvulsant evaluation of some new 2, 3, 8-trisubstituted-4(3H)-quinazoline derivatives. Bioorganic & Medicinal Chemistry Letters. 2012;22(1):327–333. doi: 10.1016/j.bmcl.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Kashaw S. K., Kashaw V., Mishra P., Jain N. K., Stables J. P. Synthesis, anticonvulsant and CNS depressant activity of some new bioactive 1-(4-substituted-phenyl)-3-(4-oxo-2-phenyl/ethyl-4H-quinazolin-3-yl)-urea. European Journal of Medicinal Chemistry. 2009;44(11):4335–4343. doi: 10.1016/j.ejmech.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Cao S. L., Feng Y. P., Jiang Y. Y., Liu S. Y., Ding G. Y., Li R. T. Synthesis and in vitro antitumor activity of 4(3H)-quinazolinone derivatives with dithiocarbamate side chains. Bioorganic and Medicinal Chemistry Letters. 2005;15(7):1915–1917. doi: 10.1016/j.bmcl.2005.01.083. [DOI] [PubMed] [Google Scholar]
- 22.Al-Obaid A. M., Abdel-Hamide S. G., El-Kashef H. A., et al. Substituted quinazolines, part 3. Synthesis, in vitro antitumor activity and molecular modeling study of certain 2-thieno-4(3H)-quinazolinone analogs. European Journal of Medicinal Chemistry. 2009;44(6):2379–2391. doi: 10.1016/j.ejmech.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Bansal E., Srivastava V. K., Kumar A. Newer substituted β-aminonaphthalenes as potent anti-inflammatory agents. Arzneimittel-Forschung/Drug Research. 2000;50(11):1009–1014. doi: 10.1055/s-0031-1300325. [DOI] [PubMed] [Google Scholar]
- 24.Srivastava S. K., Srivastava S. L., Srivastava S. D. Synthesis of new 2-chlorophenothiazinothiadiazol-2-oxoazetidines: antimicrobial and antiinflammatory agents. Indian Journal of Chemistry B. 2000;39(6):464–467. [Google Scholar]
- 25.Kumar A., Jaju B. P., Sinha J. N. 1-(2-carboxyhenyl)-3-chloro-4-aryl-azetidin-2-ones as potent anti-inflammatory agents. Indian Journal of Pharmaceutical Sciences. 1990;52(6):257–260. [Google Scholar]
- 26.Yadav R., Srivastava S. D., Srivastava S. K. Synthesis, antimicrobial and antiinflammatory activities of 4-oxothiazolidines and their 5-arylidenes. Indian Journal of Chemistry B. 2005;44(6):1262–1266. [Google Scholar]
- 27.Goel B., Ram T., Tyagi R., et al. 2-Substituted-3-(4-bromo-2-carboxyphenyl)-5-methyl-4-thiazolidinones as potential anti-inflammatory agents. European Journal of Medicinal Chemistry. 1999;34(3):265–269. doi: 10.1016/S0223-5234(99)80060-9. [DOI] [Google Scholar]
- 28.Bogert M. T. Researches on quinazolines (eighteenth paper), on 2, 3-dialkyl-4-quinazolones and the products obtained by alkylating 2-alkyl-4-quinazolones (2-alkyl-4-hydroxy quinazolones) Journal of the American Chemical Society. 1907;29(4):517–536. [Google Scholar]
- 29.Winter C. A., Risley E. A., Nuss G. W. Carrageenan induced oedema in hind paw of the rat an as assay for antiinflammatory drugs. Proceedings of the Society for Experimental Biology and Medicine. 1962;111:544–550. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 30.Berkowitz B. A., Finck A. D., Ngai S. H. Nitrous oxide analgesia: reversal by naloxone and development of tolerance. Pharmacology and Experimental Therapeutics. 1977;203:539–547. [PubMed] [Google Scholar]