Abstract
Purpose of the study
Alveolar-capillary leakage of proteinaceous fluid impairs alveolar ventilation and surfactant function and decreases lung compliance in acute lung injury. We investigated the correlation between lung function and total protein levels in bronchoalveolar lavage fluid (BALF) of ventilated, lavaged surfactant-deficient rabbits treated with various clinical and synthetic lung surfactant preparations.
Materials and Methods
109 Ventilated, young adult New Zealand White rabbits underwent lung lavage to induce surfactant-deficiency (PaO2 <100 torr in 100% O2), were treated with a clinical surfactant or a synthetic surfactant preparation with surfactant protein B (SP-B) and/or surfactant protein C (SP-C) analogs, and mechanically ventilated for 120 min. Total protein levels in postmortem BALF were correlated with arterial PO2 and dynamic lung compliance values at 120 min post-surfactant treatment.
Results
Repeated lung lavages decreased mean PaO2 values from 540 to 58 torr and lung compliance from 0.64 to 0.33 mL/kg/cm H2O. Two hours after surfactant therapy and mechanical ventilation, mean PaO2 values had increased to 346 torr and lung compliance to 0.44 mL/kg/cm H2O. Eighty-six rabbits (79%) responded to surfactant therapy with an increase in PaO2 to values >200 torr. Fourteen non-responders received inactive surfactant preparations. BALF protein levels were inversely correlated with PaO2 and lung compliance (P<0.001). Surfactant preparations containing both SP-B and SP-C proteins or peptide analogs outperformed single protein/peptide preparations.
Conclusions
Clinical and synthetic surfactant therapy reduces alveolar-capillary protein leakage in surfactant-deficient rabbits. Surfactant preparations with both SP-B and SP-C (analogs) were more efficient than preparations with SP-B or SP-C alone.
Keywords: Alveolar-capillary leakage, bronchoalveolar lavage fluid, lung function, protein, surfactant
INTRODUCTION
Lung surfactant is a mixture of lipids and proteins that is critical for normal breathing due to its ability to prevent alveolar collapse during expiration by reducing alveolar surface tension to extremely low values. Surfactant is synthesized and secreted into the alveolar fluid by alveolar type II cells and consists of approximately 80% phospholipids, 10% neutral lipids, and 10% proteins [1,2]. Although dipalmitoyl phosphatidylcholine (DPPC) and phosphatidylglycerol (POPG) constitute the principal phospholipid components in lung surfactant, its biophysical activities largely depend on the presence of the hydrophobic surfactant protein B (SP-B) and, to a lesser degree, the extremely hydrophobic surfactant protein C (SP-C) [3–5]. Hereditary SP-B deficiency is lethal in humans [6], while mutations in the SP-C gene may cause interstitial lung disease and increase susceptibility to infection [7]. The introduction of surfactant therapy using bovine or porcine lung extracts surfactant extracts, which contain only polar lipids and native SP-B and SP-C, has greatly improved the therapeutic outcomes of neonates with respiratory distress syndrome (RDS).
More recently, synthetic lung surfactant is receiving interest because of its potential to increase the efficacy and safety of neonatal surfactant treatment [8, 9] and a possible supportive role in the treatment of acute respiratory distress syndrome (ARDS) [10, 11]. Synthetic lung surfactant preparations with SP-B and SP-C peptide analogs mixed in synthetic lipid mixtures have significant pharmaceutical advantages relative to animal-derived surfactant drugs because of greater purity, better compositional reproducibility, easier manufacturing quality control, and significant scale-up economy for treating respiratory problems. Our group has developed various highly active SP-B and SP-C analogs. Mini-B (MB) [12] and Super Mini-B (SMB) [13] are 34- and 41-residue SP-B analogs based on the primary sequence, tertiary folding and quaternary association properties of the 79-residue native SP-B protein and have shown excellent surface activity, as single surfactant peptide in various lipid mixtures, in in vitro studies and in surfactant-deficient animal models [12, 13]. Native SP-C (35 residues) has a N-terminal region with two cysteines covalently linked to palmitoyl moieties and an α-helical C-terminal region that is highly enriched in valine, leucine and isoleucine residues [5, 14]. SP-Cff uses human SP-C as a template, but the adjacent palmitoylcysteine groups are replaced with phenylalanines [14, 15]. In SP-C33 the palmitoylcysteine groups are replaced with serines and the poly-valines in the α-helix are substituted with multiple leucines [16, 17]. In SP-Css ion-lock 1 the palmitoylcysteine groups are also replaced by with serines and two valines in the α-helix are swapped with a single salt-bridge (with Glu−-20 and Lys+-24 insertions) to stabilize the α-helix [15]. In vitro and in vivo studies have confirmed excellent surface activity of SP-C33 and SP-Css ion-lock 1, but disappointing results with SP-Cff [14–17].
Our hypothesis was that an effective clinical or synthetic lung surfactant not only would improve oxygenation and lung compliance, but also reduce capillary-alveolar leakage of proteinaceous fluid and lung edema in respiratory disorders characterized by surfactant-deficiency, such as RDS in preterm infants, or ARDS in children and adults. We tested this hypothesis in a large group of ventilated, surfactant-deficient rabbits, treated with clinical surfactant, synthetic lung surfactant with various (in)active SP-B and SP-C analogs, and surfactant lipids alone.
MATERIALS AND METHODS
Materials
Peptide synthesis reagents were purchased from Applied Biosystems (Foster City, CA), high performance liquid chromatography (HPLC) solvents from Fisher Chemical Co. (Pittsburgh, PA), and all other chemicals from Sigma Chemical Co. (St. Louis, MO) and Aldrich Chemical Co. (Milwaukee, WI). DPPC, palmitoyloleoyl-phosphatidylcholine (POPC) and POPG were from Avanti Polar Lipids (Alabaster, AL). Lowry assay was from Sigma (St. Louis, MO). The clinical surfactant Infasurf® (Calfactant), a bovine lung lavage extract, was a generous gift of Ony Inc (Amherst, NY), and the clinical surfactant Curosurf® (Poractant Alfa), a porcine lung extract, was a generous gift from Chiesi Farmaceutici (Parma, Italy). Young adult New Zealand White rabbits, weighing 1.0–1.3 kg, were obtained from I.F.P.S. (Norco, CA).
Synthesis, preparation, and surfactometry of synthetic surfactant preparations
SP-B and SP-C peptides were synthesized on a Symphony Multiple Peptide Synthesizer (Protein Technologies, Tucson, AZ) with standard Fmoc chemistry, purified by reverse phase HPLC, and had their molecular weights verified by MALDI-TOF [12–19]. Synthetic surfactant preparations were formulated by mixing DPPC:POPC:POPG at a 5:3:2 weight ratio [20] with 3% of native SP-B or the SP-B peptides MB [12] or SMB [13] or an inactive SP-B “mutant” thereof; 3% of the SP-C peptides SP-Cff [14, 15], SP-C33 [16, 17], or SP-Css ion–lock 1 [15], or an inactive “mutant” thereof; 1.5% each of a representative of the SP-B and SP-C peptide groups (MB + SP-C33, SMB + SP-C33, SP-Cff, or SP-Css ion-lock 1); or surfactant lipids alone. All surfactant preparations were formulated at a concentration of 35 mg phospholipids/mL. Infasurf®, which contains 35 mg/ml of phospholipids with 0.74% of SP-B and 1.08% of SP-C, and Curosurf®, which contains 80 mg/ml of phospholipids with 0.27–0.49% of SP-B and 0.67–1.55% of SP-C, were used as clinical surfactant [21, 22].
After formulation of the surfactant preparations, adsorption and dynamic surface tension lowering ability of all surfactant preparations were measured with a captive bubble surfactometer at physiological cycling rate, area compression, temperature, and humidity [13, 20, 23]. Surfactant preparations with minimum surface tension values < 2 mN/m were considered to be surface-active and those > 2 mN/m as surface-inactive.
Animal experiments
The animal experiments were performed with approval of the Animal Care and Use Committee at the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center and all procedures and anesthesia were in accordance with the American Veterinary Medical Association (AMVA) Guidelines.
Anesthesia, surgery, lavage, ventilation, and monitoring used in this study are the same as previously described [15, 18, 19]. Briefly, young adult New Zealand white rabbits (weight 1.0–1.3 kg) received anesthesia via a percutaneous intravenous line, were intubated and ventilated, and had a carotid arterial line inserted. Heart rate, arterial blood pressures and rectal temperature were monitored continuously (Labchart® Pro, ADInstruments Inc., Colorado Springs, CO, USA). Airway flow and pressures and tidal volume were monitored continuously with a pneumotachograph connected to the endotracheal tube and a pneumotach system (Hans Rudolph Inc., Kansas City, MO, USA). Maintenance fluid was provided by a continuous infusion of Lactated Ringer’s solution at a rate of 10 mL/kg/h.
After stabilization on the ventilator, surfactant deficiency was induced by saline lung lavages. When the partial pressure of oxygen in arterial blood (PaO2) was >500 torr at a peak inspiratory pressure <15 cm H2O in 100% oxygen (FiO2=1.0), the rabbits underwent repeated saline lung lavages until the PaO2 dropped below 100 torr (average 3.75 lavages of 30 mL of normal saline, temperature 37°C, average fluid recovery of 89%). When the PaO2 was stable at <100 torr, an experimental synthetic surfactant (with synthetic SP-B and/or SP-C analogs) or a clinical surfactant was instilled into the trachea at a dose of 100 mg/kg body weight and a concentration of 35 mg/mL (Curosurf at 80 mg/mL). All rabbits were ventilated with a Harvard volume-controlled animal ventilator (tidal volume 7.5 ml/kg, positive end-expiratory pressure of 3 cm H2O, inspiratory/ expiratory ratio of 1:2, FiO2 of 1.0, and a respiratory rate to maintain the PaCO2 at ~40 mmHg). Airway flow and pressures and tidal volume were monitored continuously with a pneumotachograph connected to the endotracheal tube and a pneumotach system (Hans Rudolph Inc., Kansas City, MO, USA). Respiratory function was followed by measurements of arterial pH and blood gases and dynamic lung compliance at 15 min intervals. Dynamic lung compliance was calculated by dividing tidal volume/kg body weight by changes in airway pressure (peak inspiratory pressure minus positive end-expiratory pressure) (mL/kg/cm H2O).
Animals were sacrificed 120 min after surfactant administration with an overdose of intravenous pentobarbital. Postmortem lung lavages were performed 3 times with 30 mL of normal saline. End points were oxygenation and dynamic lung compliance at 120 min after surfactant administration and total protein content of post-mortem collected bronchoalveolar lavage fluid (BALF).
Protein measurements of BALF
Total protein content of bronchoalveolar lavage fluid (BALF) collected during the first 30 mL lung lavage to induce surfactant deficiency and the first 30 mL postmortem lavage was measured with a Lowry assay using human albumin as a standard (n=3–5 assays per sample) [24].
Data analysis
Data are expressed as means ± standard deviation (SD). Correlations between oxygenation, lung compliance and BALF protein content were estimated using linear regression analysis (SPSS Statistics 22, IBM, Armonk, NY). A P value <0.05 was considered to indicate a significant difference.
RESULTS
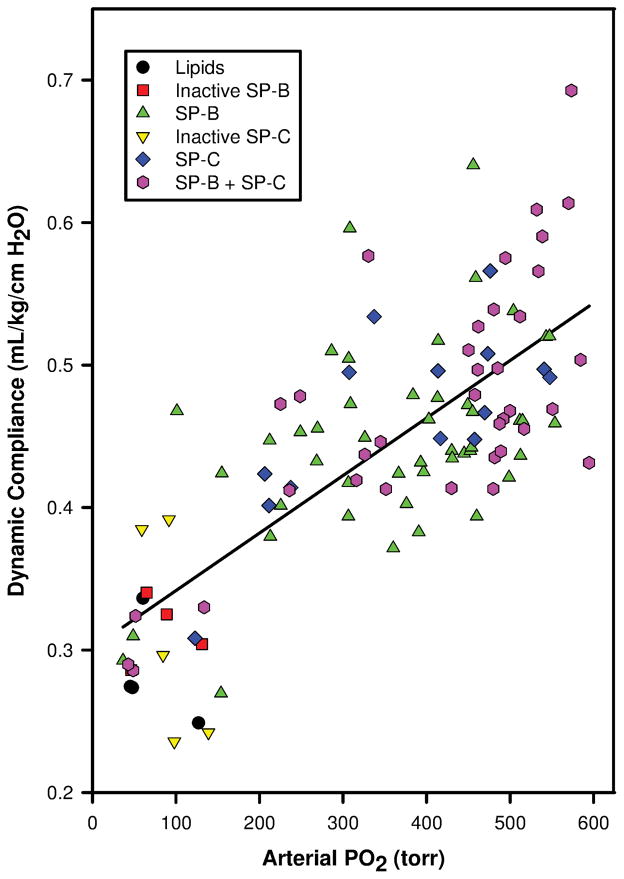
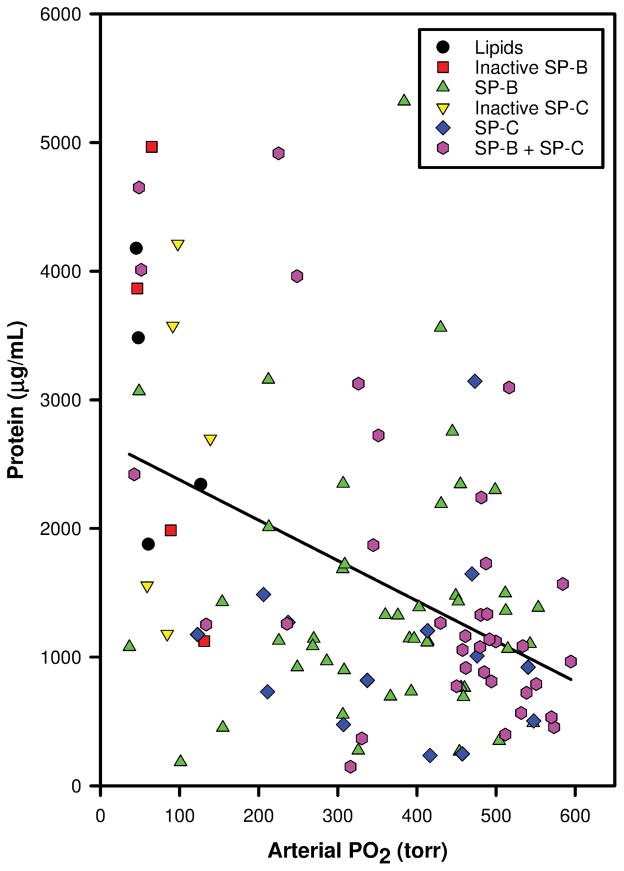
Data on oxygenation, dynamic lung compliance and BALF protein of 109 surfactant-deficient young adult rabbits at 120 min post-surfactant instillation were analyzed. During the first set of lavages to induce surfactant-deficiency, mean ± SD PaO2 values dropped from 540 ± 37 to 58 ± 19 torr and dynamic lung compliances decreased from 0.64 ± 0.17 to 0.33 ± 0.05 mL/kg/cmH2O. After intratracheal surfactant instillation, PaO2 values and dynamic lung compliance increased rapidly (Table 1). Fifty rabbits received surfactant preparations with native SP-B (n=6) or SP-B analogs (16 MB varieties, 23 SMB varieties, 5 SP-B “mutants”), 19 received SP-C analogs (9 SP-C33, 4 SP-Css ion-lock, 1 SP-Cff, and 5 SP-C “mutants”), 36 received surfactant with SP-B and SP-C proteins/analogs (8 Infasurf, 7 Curosurf, 7 SMB + SP-C33, 3 SMB + SP-Cff, 7 MB + SP-Css ion-lock 1, and 4 MB + SP-C33), and 4 received surfactant lipids alone. The 5 SP-B “mutants” consisted of 3 SMB and 2 MB in which cysteines were replaced by phenylalanine or serine, respectively, resulting in disruption of intramolecular disulfide bridges. The 5 SP-C “mutants” had changes in the sequence of the N-terminal segment, leading to a tendency to form β-turns instead of α-helices and thereby loss of surface activity [25]. There was a strong correlation between PaO2 and dynamic lung compliance (r=0.743, p<0.001, Fig. 1). Total protein in the BALF collected during the lavages to induce surfactant deficiency was 261 ± 148 μg/mL and had increased to 1604 ± 1173 μg/mL in the BALF collected at 120 min post-surfactant treatment (Table 1). Linear regression analysis showed a significant inverse correlation between PaO2 (Fig. 2) and dynamic compliance (Fig. 3) values and total protein values in BALF at 120 min post-surfactant treatment.
Table 1.
Arterial oxygenation (PaO2), dynamic lung compliance and bronchoalveolar lavage fluid (BALF) protein data at 120 min post-surfactant treatment in all rabbits, subdivided into those treated with surface-inactive and active surfactants. Active surfactants were subdivided by surfactant composition.
| Surfactant preparation | N | PaO2 (torr) | Dynamic Compliance (mL/kg/cm H2O) | BALF protein (μg/mL) |
|---|---|---|---|---|
| All surfactants | 109 | 346 ± 166 | 0.441 ± 0.090 | 1604 ± 1173 |
| •Inactive surfactant | 14 | 89 ± 41** | 0.318 ± 0.073** | 2237 ± 1331* |
| •Active surfactant: | 95 | 384 ± 141 | 0.459 ± 0.077 | 1511 ± 1126 |
| ◦SP-B + SP-C | 36 | 411 ± 157 | 0.474 ± 0.089 | 1604 ± 1235 |
| ◦SP-B | 45 | 366 ± 128 | 0.446 ± 0.069 | 1577 ± 1122 |
| ◦SP-C | 14 | 373 ± 136 | 0.464 ± 0.064 | 1063 ± 741 |
:P = 0.03 and
: P <0.0001 versus active surfactants
Figure 1.
Correlation between arterial oxygenation (PaO2, torr) and dynamic lung compliance (mL/kg/cm H2O) values in 109 surfactant-deficient rabbits at 120 min post-surfactant instillation (linear regression analysis, r = 0.743, P <0.001). Composition of surfactant preparations is indicated with symbols.
Figure 2.
Correlation between arterial oxygenation (PaO2, torr) and bronchoalveolar lavage fluid (BALF) protein content (μg/mL) in 109 surfactant-deficient rabbits at 120 min post-surfactant instillation (linear regression analysis, r = −0.442, P <0.001). Composition of surfactant preparations is indicated with symbols.
Figure 3.
Correlation between dynamic lung compliance (mL/kg/cmH2O) and bronchoalveolar lavage fluid (BALF) protein content (μg/mL) in 109 surfactant-deficient rabbits at 120 min post-surfactant instillation (linear regression analysis, r = −0.452, P <0.001). Composition of surfactant preparations is indicated with symbols.
Eighty-six out of 109 rabbits (79%) responded to surfactant therapy with a rapid increase in PaO2 to values >200 torr. The 23 animals that did not reach this limit were labeled as non- responders. Fourteen of the 23 non-responders (61%) received either surfactant lipids alone (n=4) or surfactant preparations with in vitro inactive SP-B (n=5) or SP-C (n=5) analogs (“inactive surfactant” group in Table 1). Nine non-responders received active SP-B (4/45 or 9%) or SP-C (1/14 or 7%) analogs, a combination of SP-B and SP-C analogs (2/21 or 10%) or clinical surfactant (2/15 or 13%). In the 86 responders total protein was 1381 ± 989 μg/mL in the postmortem BALF versus 2468 ± 1476 μg/mL in the 23 non-responders (P < 0.0001).
Correlations between lung function (oxygenation and dynamic compliance) and BALF protein values were further analyzed for each of the 3 major surfactant groups (Table 2), i.e. synthetic surfactant preparations with native SP-B or a SP-B analog, surfactant preparations with a SP-C analog, and clinical (animal-derived) and synthetic surfactant preparations with SP-B + SP-C proteins or analogs. Clinical and synthetic surfactant preparations with both SP-B and SP-C consistently demonstrated a more consistent association between oxygenation and dynamic lung compliance and BALF protein values than single peptide or protein surfactant preparations (p<0.001).
Table 2.
Correlations between arterial oxygenation (PaO2), dynamic lung compliance and bronchoalveolar lavage fluid (BALF) protein values in 3 groups of surfactant preparations, i.e. those with SP-B, those with SP-C and those with SP-B + SP-C peptides/proteins, estimated by linear regression analysis.
| Surfactant | N | r | P-value |
|---|---|---|---|
| PaO2 vs dynamic lung compliance | |||
| SP-B | 50 | 0.296 | 0.037 |
| SP-C | 19 | 0.497 | 0.030 |
| SP-B + SP-C | 36 | 0.543 | <0.001 |
| PaO2 vs BALF protein | |||
| SP-B | 50 | −0.248 | 0.082 |
| SP-C | 19 | −0.445 | 0.056 |
| SP-B + SP-C | 36 | −0.633 | <0.001 |
| Dynamic lung compliance vs BALF protein | |||
| SP-B | 50 | 0.624 | <0.001 |
| SP-C | 19 | −0.798 | <0.001 |
| SP-B + SP-C | 36 | −0.720 | <0.001 |
DISCUSSION
As expected, intratracheal instillation of surface-active surfactant preparations, consisting of SP-B, SP-C or a combination of SP-B and C analogs in synthetic lipids or clinical surfactant with native SP-B and SP-C, quickly improved both oxygenation, as shown by increasing PaO2 values, and dynamic lung compliance in a large majority of lavaged, surfactant-deficient young adult rabbits. Likewise, instillation of surfactant preparations containing surfactant lipids alone or surface-inactive SP-B or SP-C analogs did not influence lung function. Whereas pre-surfactant BALF protein values were similar among all groups, post-mortem BALF protein values were significantly higher in non-responders than in responders to surfactant therapy and there was an inverse relationship between BALF protein values and PaO2 and dynamic compliance values. Comparison of single peptide or native SP-B and single peptide SP-C preparations with clinical and synthetic surfactant preparations containing both SP-B and SP-C demonstrated better results with the latter ones. These findings suggest that double peptide (SP-B and SP-C) surfactant preparations and comparable clinical surfactants may be more effective in reducing alveolar-capillary protein leakage than single SP-B or SP-C peptide or native SP-B preparations.
A remarkable finding was the large increase in BALF protein values between the pre-surfactant and post-mortem values, a phenomenon that was not only seen and expected in animals treated with inactive surfactant preparations (negative controls) and non-responders, but also, though significantly less, in animals treated with in vitro surface active surfactant preparations. Instillation of surfactant introduces a source of proteins into the lung as it contains between 1.5 and 3% of protein/peptide, i.e. ~1.5–3 mg at a surfactant dosage of 100 mg/kg body weight (of 1.0–1.3 kg) depending on the type of surfactant used. However, the significant differences in BALF protein content between active and inactive surfactants at 120 min post-surfactant suggest that surfactant treatment does more good than harm and plays an important role in diminishing alveolar-capillary leakage. This finding suggests that repeated lung lavages lead to acute lung injury and alveolar-capillary leakage with sizeble increases in BALF protein content and that all surfactants reduce this leakage, with variable efficiency depending on the surfactant protein or peptide component(s). Inclusion of true negative controls, e.g. rabbits treated with intratracheal instillation of normal saline instead of phospholipids only, might have helped here, but in our hands lavaged, ventilated rabbits with severe respiratory failure rarely survive for 120 min without surfactant therapy.
It is well-known that high surface tension and pulmonary instability at low lung volumes induces inflammation in the lung epithelium that this inflammatory response causes alteration in the alveolar-capillary permeability barrier, leading to leakage of serum into the airspaces. Our study might have benefited from a correlation of an indicator of inflammation with both oxygenation efficiency and protein levels, but the necessary samples were not available.
Another limitation of our study was that we based our analysis and conclusions on the differences between inactive surfactants, phospholipids supplemented with one peptide and phospholipids with two peptides, and did not compare each analog we used as the numbers in the various groups differed because a mediocre surfactant was tested in a smaller number of animals than an outstanding one. We measured surface activity of all experimental surfactant preparations with captive bubble surfactometry prior to testing them in animals. Based on this in vitro surface activity, we divided the surfactants in active or inactive preps. Some “mutant” peptides were made to study structure-function relationships and served as inactive controls. The efficacy of various synthetic surfactants (Mini-B, Super Mini-B, SP-Cff, SP-C33, and SP-Css ion-lock 1) have been reported previously [12–17].
Here, we did not provide data showing that two-peptide synthetic surfactants are equivalent or superior to animal-derived surfactants (Curosurf and Infasurf) because this has been addressed by previous studies and recent studies with CHF5633 (MB + SP-C33) have once more independently confirmed this observation [26, 27].
In this study we compare the effects of surfactant preparations with 1.5% (by weight) of each of the two surfactant proteins (SP-B and SP-C, or their analogs) and preparations with 3% (by weight) of only one of the two surfactant proteins with respect to phospholipids. Weight proportions differ from molar ratios, particularly when comparing native surfactant proteins with analogs with shorter peptide segments. The quantities of SP-B protein or analogs we used represent more surface active SP-B molecules than natural surfactants, as 1.5% (by weight) of a SP-B analog already provides about 4x (half the size and twice the percentage) as many SP-B molecules as the ~0.75 % of native SP-B in Infasurf. SP-C analogs do not greatly differ in weight from native SP-C and 1.5% (by weight) of a SP-C analog therefore equally surpasses the SP-C content of a natural surfactant.
Due to a lack of publications reporting BALF proteins throughout the course of surfactant therapy, we were not able to compare our data with those of others. However, these findings should raise more awareness about the role of surfactant therapy in reducing alveolar-capillary leakage under conditions of surfactant deficiency, such as seen in preterm infants with RDS.
Acknowledgments
FUNDING
The authors gratefully acknowledge the financial support of the National Institutes of Health through grants R01HL092158 and R01ES015330. NIH had no role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, and in the preparation, review, or approval of the manuscript.
Footnotes
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Contributor Information
Rohun Gupta, Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Torrance, California, USA.
José M. Hernández-Juviel, Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Torrance, California, USA
Alan J. Waring, Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Torrance, California, USA; Departments of Pediatrics and Medicine, David Geffen School of Medicine, UCLA, Los Angeles, California, USA; and Department of Physiology & Biophysics, School of Medicine, UCI, Irvine, California, USA
Frans J. Walther, Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Torrance, California, USA; and Department of Pediatrics, David Geffen School of Medicine, UCLA, Los Angeles, California, USA
References
- 1.Goerke J. Pulmonary surfactant: functions and molecular composition. Biochim Biophys Acta. 1998;1408:79–89. doi: 10.1016/s0925-4439(98)00060-x. [DOI] [PubMed] [Google Scholar]
- 2.Perez-Gil J. Molecular interactions in pulmonary surfactant films. Biol Neonate. 2002;81:6–15. doi: 10.1159/000056765. [DOI] [PubMed] [Google Scholar]
- 3.Possmayer F. A proposed nomenclature for pulmonary surfactant-associated proteins. Am Rev Respir Dis. 1988;138:990–998. doi: 10.1164/ajrccm/138.4.990. [DOI] [PubMed] [Google Scholar]
- 4.Walther FJ, Gordon LM, Zasadzinski JA, Sherman MA, Waring AJ. Surfactant protein B and C analogues. Mol Genet Metab. 2000;71:342–351. doi: 10.1006/mgme.2000.3053. [DOI] [PubMed] [Google Scholar]
- 5.Walther FJ, Waring AJ, Sherman MA, Zasadzinski JA, Gordon LM. Hydrophobic surfactant proteins and their analogues. Neonatology. 2007;91:303–310. doi: 10.1159/000101346. [DOI] [PubMed] [Google Scholar]
- 6.Nogee LM, Garnier G, Dietz HC, Singer L, Murphy AM, deMello DE, et al. A mutation in the surfactant protein B gene responsible for fatal neonatal respiratory disease in multiple kindreds. J Clin Invest. 1994;93:1860–1863. doi: 10.1172/JCI117173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bridges JP, Xu Y, Na C-L, Wong HR, Weaver TE. Adaptation and increased susceptibility to infection associated with constitutive expression of misfolded SP-C. J Cell Biol. 2006;172:395–407. doi: 10.1083/jcb.200508016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moya FR, Gadzinowski J, Bancalari E, Salinas V, Kopelman B, Bancalari A, Kornacka MK, Merritt TA, Segal R, Schaber CJ, Tsai H, Massaro J, d’Agostino R International Surfaxin Collaborative Study Group. A multicenter, randomized, masked, comparison trial of lucinactant, colfosceril palmitate, and beractant for the prevention of respiratory distress syndrome among very preterm infants. Pediatrics. 2005;115:1018–29. doi: 10.1542/peds.2004-2183. [DOI] [PubMed] [Google Scholar]
- 9.Sinha SK, Lacaze-Masmonteil T, Valls i Soler A, Wiswell TE, Gadzinowski J, Hajdu J, Bernstein G, Sanchez-Luna M, Segal R, Schaber CJ, Massaro J, d’Agostino R Surfaxin Therapy Against Respiratory Distress Syndrome Collaborative Group. A multicenter, randomized, controlled trial of lucinactant versus poractant alfa among very premature infants at high risk for respiratory distress syndrome. Pediatrics. 2005;115:1030–1038.23. doi: 10.1542/peds.2004-2231. [DOI] [PubMed] [Google Scholar]
- 10.Willson DF, Chess PR, Notter RH. Surfactant for pediatric acute lung injury. Pediatr Clin North Am. 2008 Jun;55(3):545–575. doi: 10.1016/j.pcl.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raghavendran K, Willson D, Notter RH. Surfactant therapy for acute lung injury and acute respiratory distress syndrome. Crit Care Clin. 2011;27:525–559. doi: 10.1016/j.ccc.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waring AJ, Walther FJ, Gordon LM, Hernandez-Juviel JM, Hong T, Sherman MA, et al. The role of charged amphipathic helices in the structure and function of surfactant protein B. J Pept Res. 2005;66:364–374. doi: 10.1111/j.1399-3011.2005.00300.x. [DOI] [PubMed] [Google Scholar]
- 13.Walther FJ, Waring AJ, Hernandez-Juviel JM, Gordon LM, Wang Z, Jung C-L, et al. Critical structural and functional roles for the N-terminal insertion sequence in surfactant protein B analogs. PLoS One. 2010;5:e8672. doi: 10.1371/journal.pone.0008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walther FJ, Gordon LM, Zasadzinski JA, Sherman MA, Waring AJ. Surfactant protein B and C analogues. Mol Genet Metab. 2000;71:342–351. doi: 10.1006/mgme.2000.3053. [DOI] [PubMed] [Google Scholar]
- 15.Walther FJ, Waring AJ, Hernández-Juviel JM, Ruchala P, Wang Z, Notter RH, et al. Surfactant protein C peptides with salt-bridges (“ion-locks”) promote high surfactant activities by mimicking the α-helix and membrane topography of the native protein. PeerJ. 2014;2:e485. doi: 10.7717/peerj.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johansson J, Some M, Linderholm BM, Almlén A, Curstedt T, Robertson B. A synthetic surfactant based on a poly-Leu SP-C analog and phospholipids: effects on tidal volumes and lung gas volumes in ventilated immature newborn rabbits. J Appl Physiol. 2003;95:2055–2063. doi: 10.1152/japplphysiol.00153.2003. [DOI] [PubMed] [Google Scholar]
- 17.Almlén A, Walther FJ, Waring AJ, Robertson B, Johansson J, Curstedt T. Synthetic surfactant based on analogues of SP-B and SP-C is superior to single-peptide surfactants in ventilated premature rabbits. Neonatology. 2010;98:91–99. doi: 10.1159/000276980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walther FJ, Hernandez-Juviel JM, Waring AJ. Aerosol delivery of synthetic lung surfactant. PeerJ. 2014;2:e403. doi: 10.7717/peerj.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walther FJ, Hernández-Juviel JM, Gordon LM, Waring AJ. Synthetic surfactant containing SP-B and SP-C mimics is superior to single-peptide formulations in rabbits with chemical acute lung injury. PeerJ. 2014 May 22;2:e393. doi: 10.7717/peerj.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walther FJ, Hernández-Juviel JM, Gordon LM, Waring AJ, Stenger P, Zasadzinski JA. Comparison of three lipid formulations for synthetic surfactant with a surfactant protein B analog. Exp Lung Res. 2005;31:563–579. doi: 10.1080/019021490951531. [DOI] [PubMed] [Google Scholar]
- 21.Bernhard W, Mottaghian J, Gebert A, Rau GA, von Der Hardt H, Poets CF. Commercial versus native surfactants. Surface activity, molecular components, and the effect of calcium. Am J Respir Crit Care Med. 2000;162:1524–33. doi: 10.1164/ajrccm.162.4.9908104. [DOI] [PubMed] [Google Scholar]
- 22.Taeusch HW, Lu K, Ramierez-Schrempp D. Improving pulmonary surfactants. Acta Pharmacol Sin. 2002;23 (Suppl):11–15. [Google Scholar]
- 23.Schürch S, Bachofen H, Possmayer F. Surface activity in situ, in vivo, and in the captive bubble surfactometer. Comp Biochem Physiol A Mol Integr Physiol. 2001;129:195–207. doi: 10.1016/s1095-6433(01)00316-6. [DOI] [PubMed] [Google Scholar]
- 24.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin Phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 25.Plasencia I, Rivas L, Casals C, Keough KM, Pérez-Gil J. Intrinsic structural differences in the N-terminal segment of pulmonary surfactant protein SP-C from different species. Comp Biochem Physiol A Mol Integr Physiol. 2001;129:129–139. doi: 10.1016/s1095-6433(01)00310-5. [DOI] [PubMed] [Google Scholar]
- 26.Sato A, Ikegami M. SP-B and SP-C containing new synthetic surfactant for treatment of extremely immature lamb lung. PLoS One. 2012;7(7):e39392. doi: 10.1371/journal.pone.0039392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salvesen B, Curstedt T, Mollnes TE, Saugstad OD. Effects of natural versus synthetic surfactant with SP-B and SP-C analogs in a porcine model of meconium aspiration syndrome. Neonatology. 2014;105:128–135. doi: 10.1159/000356065. [DOI] [PubMed] [Google Scholar]