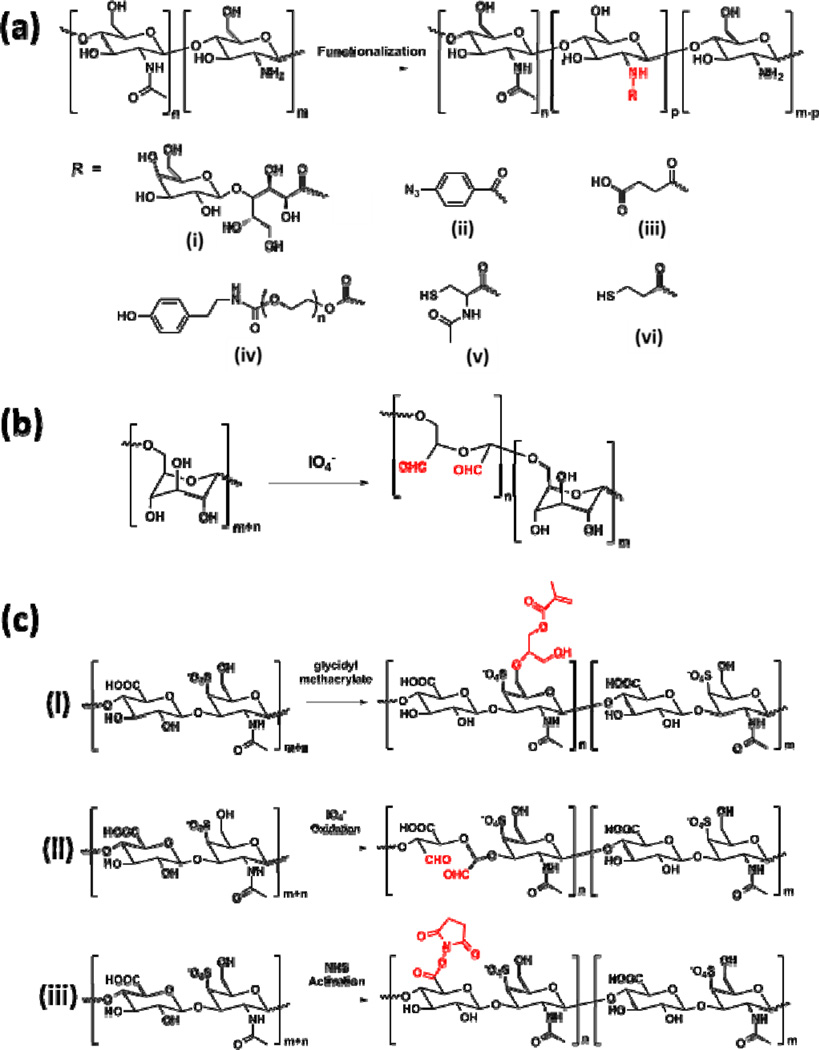
Figure 3.
Representative chemical structures of polysaccharide-based sealants. (A) Deacetylation of chitin generates chitosan with different degrees of acetylation, and various chemically modified chitosan derivatives are obtained by reacting chitosan with (i) lactobionic acid, (ii) 4-azidobenzoic acid, (iii) succinic anhydride, ((iv) PEG oligomers, (v) N-acetylcysteine, and (vi) 3-mercaptopropionic acid at the amine site [51–57]. (B) Preparation of aldehyde-containing dextran via selective partial oxidation by periodide [60, 61]. (C) Chemical modification of chondroitin sulfate to introduce (i) methacrylate groups via reacting with glycidyl methacrylate [63], (ii) aldehyde groups via the oxidation reaction by periodide [64], and (iii) NHS-activated ester groups [66].