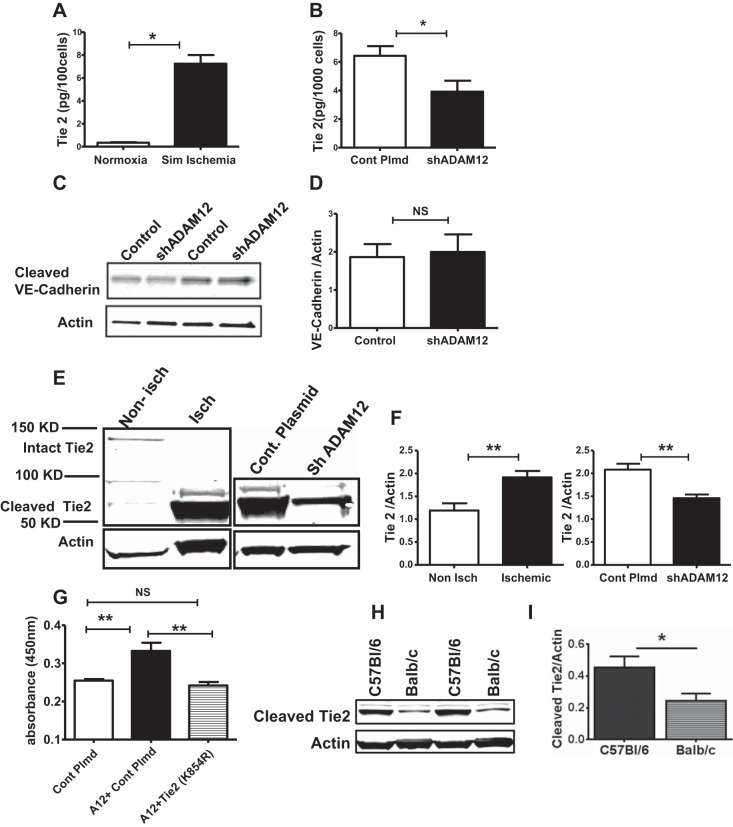
Fig. 7.
ADAM12 regulates tyrosine kinase with Ig-like and EGF-like domain 2 (Tie2) shedding, and ADAM12-induced EC proliferation is Tie2 dependent. A: exposure of HUVECs to simulated ischemia increased Tie2 shedding (n = 4/group, *P < 0.01). B: ADAM12 “knock down” (shADAM12) decreased HUVEC Tie2 shedding in simulated ischemia (n = 6/group, *P < 0.05). C: the levels of the cleaved vascular endothelial (VE)-cadherin were not different between control and ADAM12 knockdown HUVECS (n = 6/group, NS = P > 0.05). D: quantification of the bands in C. E, left: lysates from ischemic mouse hindlimbs show higher levels of cleaved Tie2 compared with nonischemic hindlimbs; right: lysates from ischemic hindlimbs, in which ADAM12 was “knocked down” (shADAM12), show decreased cleaved Tie2 compared with ischemic hindlimbs that received control plasmid (Cont Plmd). F, left: quantification of blot represented in E, left; right, quantification of the blot represented in E, right (n = 4 for nonischemic, n = 5 for ischemic, **P < 0.01). G: kinase dead Tie2 (lysine to arginine at amino acid 854 or K854R) abolishes ADAM12 (A12)-induced HUVEC proliferation (n = 7–8/group, **P < 0.01, NS = P > 0.05). H: lysates from ischemic C57Bl/6 hindlimb muscles show higher levels of cleaved Tie2 compared with that from Balb/c. I: quantification of band in H (n = 4/group, *P < 0.05).