ABSTRACT
We report the first case of methicillin-resistant Staphylococcus aureus (MRSA) keratitis in a racehorse. A 5-year-old mare developed punctate keratitis after racing. The corneal ulcer continued to expand despite ophthalmic antimicrobial therapy. On day 6, a conjunctival graft surgery was performed. The mare was euthanized, following colitis and laminitis development on day 10. MRSA was isolated from the corneal swab taken at the time of euthanasia. Immunohistochemical analysis demonstrated gram-positive and anti-S. aureus monoclonal antibody-positive cocci infiltration of the corneal stroma; and a diagnosis of MRSA ulcerative keratitis was made. An ophthalmic antimicrobial against the isolated MRSA did not improve the ocular lesion. The MRSA strain was found to be staphylococcal cassette chromosome mec type II, a strain frequently isolated from humans in Japan.
Keywords: corneal ulcer, horse, keratitis, methicillin-resistant Staphylococcus aureus
A number of methicillin-resistant Staphylococcus aureus (MRSA) strains are multidrug resistant bacteria. They are resistant not only to β-lactam antimicrobials, but also other antimicrobials such as aminoglycosides, tetracyclines, and fluoroquinolones [6]. In horses, various types of MRSA infections have been reported, including those of the skin and soft tissues [14], joints [4, 18], mastitis [18], metritis [2], and pneumonia [18]. MRSA keratitis has been reported in humans [10, 12] and a dog [17], but not in horses. Here we report on the identification of MRSA keratitis in a Thoroughbred racehorse that was confirmed by microbiological, histopathological, and immunohistochemical analyses.
A 5-year-old mare was found to have acute swelling of the eyelids and mucous discharge from the left eye, on the day following participation in a race. The mare was examined at the Racehorse Hospital, Ritto Training Center, Japan Racing Association, and superficial punctate keratitis was diagnosed on the basis a positive fluorescein staining test. Treatment of ophthalmic ofloxacin instillation every 12 hr (Tarivid ophthalmic solution 0.3%; Santen Pharmaceutical Co., Ltd., Osaka, Japan) and intravenous flunixin meglumine (1.0 mg/kg every 12 hr; Banamine injection 5%; DS Pharma Animal Health Co., Ltd., Osaka, Japan) were administered on day 1. The corneal condition deteriorated on day 2, and a deep ulcer of approximately 10 mm was found in the center of the cornea in addition to miosis indicating the development of anterior uveitis (Fig. 1). Topical autologous serum, ophthalmic ofloxacin, and ophthalmic tobramycin (Tobracin ophthalmic solution 0.3%; Nitto Medic Co., Ltd., Toyama, Japan) were administered every 2–4 hr in addition to ophthalmic atropine that was administered every 12 hr (Nitten atropine ophthalmic solution 1%; NITTEN Pharmaceutical Co., Ltd., Nagoya, Japan). On day 3, the cornea was severely edematous and the ulcer had grown 15 mm in diameter. Furthermore, hypopyon was present. A subpalpebral catheter was inserted under the upper eyelid, and 0.5% ophthalmic chloramphenicol was administered every 4 hr (Chloramphenicol ophthalmic solution 0.5%; Nitto Medic Co., Ltd.) in addition to the aforementioned ophthalmic treatments. Therapy remained unsuccessful, and further expansion of the ulcer to a diameter of 20 mm was observed. Negative fluorescein staining was observed at the center of the ulcer indicating descemetocele on day 5 (Fig. 2). Due to the severity of these symptoms, a conjunctival graft and temporary tarsorrhaphy were performed under general anesthesia on day 6, according to a previously reported guideline [1]. Following surgery, ophthalmic ofloxacin, tobramycin, and chloramphenicol were instilled every 4–6 hr and flunixin meglumine was intravenously administered every 12 hr. The mare developed circulatory shock accompanied with depression, anorexia, severe watery diarrhea, and acute laminitis on day 9. We diagnosed severe enterocolitis and laminitis which are frequently reported as post-surgical complications in horses [8, 13]. Due to the mare’s inability to stand, she was euthanized, as per veterinarian’s decision, on day 10.
Fig. 1.
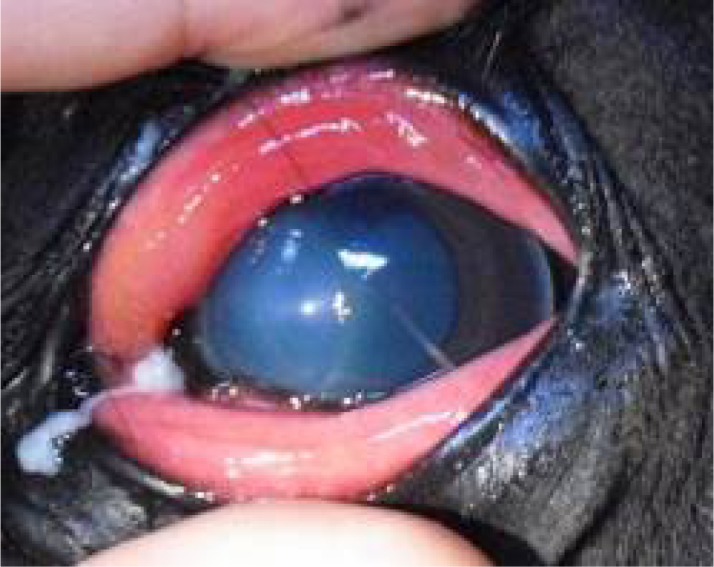
Visual image of the cornea on day 2. An approximately 10-mm ulcer was found in the cornea in addition to conjunctival edema and mucous discharge.
Fig. 2.

Visual image of the corneal ulcer on day 5. Fluorescein staining revealed a corneal ulcer of 20 mm in diameter. Negative staining at the center of the ulcer indicated descemetocele.
Necropsy was performed immediately after euthanasia. Severe corneal edema, ulceration (diameter, 20 mm), and descemetocele were observed following removal of the grafted conjunctive tissue. The vertical section of the affected eye revealed hemorrhage into the aqueous humor and severe iritis with hyperemia, congestion, and hemorrhage of the iris. General histopathology and immunohistochemistry were performed according to routine procedures. The histopathological examination revealed total loss of the corneal epithelium and the stroma corresponding to the macroscopic regions of the descemetocele. In addition, polymorphonuclear neutrophil (PMN) infiltration, red blood cell contamination (not hemorrhage), and proliferating basophilic cocci were observed in the deep corneal stroma (Fig. 3a). Gram staining revealed the presence only of Gram-positive cocci. No fungal structures were detected by periodic acid-Schiff staining. Immunohistochemistry using a monoclonal anti-S. aureus antibody (ab37644, Abcam K.K., Tokyo, Japan) revealed Gram-positive cocci and the cytoplasm of PMNs to be positive. This result suggested that PMNs reacted in a phagocytic manner with S. aureus (Fig. 3b).
Fig. 3.
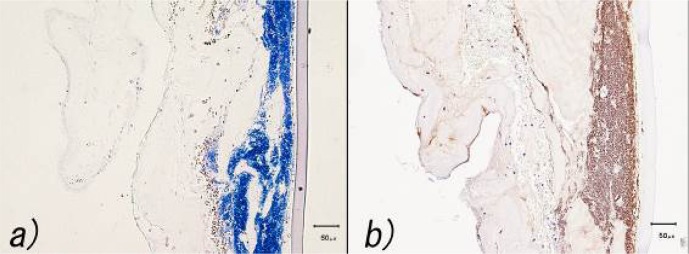
Histopathological examination of the corneal lesion. a) Gram staining demonstrated Gram-positive cocci. Hemorrhage and infiltration of polymorphonuclear neutrophils were observed in the stroma. b) Immunohistochemical staining with a monoclonal anti-Staphylococcus aureus antibody revealed Gram-positive cocci and the cytoplasm of the polymorphonuclear neutrophils to be positive.
For bacterial isolation, corneal swabs taken after euthanasia were cultured using Columbia agar supplemented with 5% horse blood. The agar plates were incubated at 37°C for 24 hr under aerobic conditions with 5% CO2. Colonies were picked from the plate and subcultured. On the basis of colony morphology, Gram staining, and biochemical characteristics, the isolated bacterium was identified as S. aureus, according to the API Biochemical Identification Kit (Sysmex bioMerieux, Tokyo, Japan). This was consistent with the results of the histopathological and immunohistochemical examinations. No other microbes, or fungi, were isolated.
The minimum inhibitory concentration (MIC) of oxacillin for the isolated S. aureus was determined using Etest (Sysmex bioMérieux) with Mueller-Hinton agar (Becton, Dickinson and Co., Tokyo, Japan) in accordance with manufacturer’s instruction. The MIC of oxacillin for the bacterial isolate was >256 µg/ml; thus, the isolate was identified to be MRSA, according to the criteria of the Clinical and Laboratory Standards Institute: S. aureus is identified as MRSA when the MIC of oxacillin is ≥4 µg/ml [3]. On the bases of microbiological and histological examination at autopsy, a final diagnosis of MRSA keratitis was made.
Antimicrobial susceptibility tests were performed using disc diffusion (BBL Sensi-Discs; Becton Dickinson and Co., Franklin Lakes, NJ, U.S.A.). The isolated strain was found to be susceptible to chloramphenicol and vancomycin and resistant to ampicillin, cephalothin, fosfomycin, gentamicin, imipenem, minocycline, ofloxacin, tetracycline, and tobramycin.
The isolate was subjected to staphylococcal cassette chromosome mec (SCCmec) typing using multiplex polymerase chain reaction (PCR), as previously reported [7]. Based on SCCmec typing, the strain was classified as SCCmec type II. The SCCmec type II strain is a major strain primarily found in healthcare-associated MRSA infection in humans in Japan [5, 11, 19]. In North America, MRSA of SCCmec type IV has been frequently isolated from horses and horse personnel, suggesting to the probability of a zoonotic transmission of MRSA isolates between horses and humans [9, 18]. Though we were not able to determine the transmission route, it is possible that hospital or stable personnel were carriers of the MRSA strain identified in the present study which in turn resulted in the mare’s lesion. To clarify the route of infection, further surveillance of MRSA colonization in Japan among personnel, including veterinarians, groomers, and racehorses, is needed.
In previous studies describing the detection of MRSA keratitis in humans [10, 15, 16] and a dog [17], the isolated strains were found to be susceptible to chloramphenicol and vancomycin, and the therapy of these antimicrobials demonstrated good results. Though the isolated MRSA strain was susceptible to chloramphenicol in this case, administration of an ophthalmic solution did not improve the mare’s condition. We cannot explain the unsuccessful result in the present case. More cases are needed to establish the therapy for MRSA keratitis in horses. This case highlights the importance of paying close attention to the selection and administration of antimicrobials in cases of MRSA keratitis in horses.
References
- 1.Alison B.C. 2011. Diseases and surgery of the cornea. pp. 181–266. In: Equine Ophthalmology, 2nd ed. (Gilger, B.C.), Elsevier, St. Louis. [Google Scholar]
- 2.Anzai T., Kamada M., Kanemaru T., Sugita S., Shimizu A., Higuchi T. 1996. Isolation of methicillin-resistant Staphylococcus aureus (MRSA) from mares with metritis and its zooepidemiology. J. Equine Sci. 7: 7–11. [Google Scholar]
- 3.Clinical and Laboratory Standards Institute 2005. Performance standards for antimicrobial susceptibility testing. 15th informational supplement. Approved standard M100-S15. Wayne. [Google Scholar]
- 4.Cuny C., Kuemmerle J., Stanek C., Willey B., Strommenger B., Witte W. 2006. Emergence of MRSA infections in horses in a veterinary hospital: strain characterisation and comparison with MRSA from humans. Euro Surveill. 11: 44–47. [PubMed] [Google Scholar]
- 5.Hisata K., Kuwahara-Arai K., Yamanoto M., Ito T., Nakatomi Y., Cui L., Baba T., Terasawa M., Sotozono C., Kinoshita S., Yamashiro Y., Hiramatsu K. 2005. Dissemination of methicillin-resistant staphylococci among healthy Japanese children. J. Clin. Microbiol. 43: 3364–3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kali A., Stephen S., Umadevi S., Kumar S., Joseph N.M., Srirangaraj S. 2013. Changing trends in resistance pattern of methicillin resistant Staphylococcus aureus. J. Clin. Diagn. Res. 7: 1979–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kondo Y., Ito T., Ma X.X., Watanabe S., Kreiswirth B.N., Etienne J., Hiramatsu K. 2007. Combination of multiplex PCRs for staphylococcal cassette chromosome mec type assignment: rapid identification system for mec, ccr, and major differences in junkyard regions. Antimicrob. Agents Chemother. 51: 264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larsen J., Dolvik N.I., Teige J., Jr 1996. Acute post-treatment enterocolitis in 13 horses treated in a Norwegian surgical ward. Acta Vet. Scand. 37: 203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin Y., Barker E., Kislow J., Kaldhone P., Stemper M.E., Pantrangi M., Moore F.M., Hall M., Fritsche T.R., Novicki T., Foley S.L., Shukla S.K. 2011. Evidence of multiple virulence subtypes in nosocomial and community-associated MRSA genotypes in companion animals from the upper midwestern and northeastern United States. Clin. Med. Res. 9: 7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyamoto T., Eguchi H., Tserennadmid E., Mitamura-Aizawa S., Hotta F., Mitamura Y. 2013. Methicillin-resistant Staphylococcus aureus keratitis after descemet’s stripping automated endothelial keratoplasty. Case Rep. Ophthalmol. 4: 269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakaminami H., Noguchi N., Ito A., Ikeda M., Utsumi K., Maruyama H., Sakamoto H., Senoo M., Takasato Y., Nishinarita S. 2014. Characterization of methicillin-resistant Staphylococcus aureus isolated from tertiary care hospitals in Tokyo, Japan. J. Infect. Chemother. 20: 512–515. [DOI] [PubMed] [Google Scholar]
- 12.Ong S.J., Huang Y.C., Tan H.Y., Ma D.H., Lin H.C., Yeh L.K., Chen P.Y., Chen H.C., Chuang C.C., Chang C.J., Hsiao C.H. 2013. Staphylococcus aureus keratitis: a review of hospital cases. PLoS ONE 8: e80119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parsons C.S., Orsini J.A., Krafty R., Capewell L., Boston R. 2007. Risk factors for development of acute laminitis in horses during hospitalization: 73 cases (1997–2004). J. Am. Vet. Med. Assoc. 230: 885–889. [DOI] [PubMed] [Google Scholar]
- 14.Seguin J.C., Walker R.D., Caron J.P., Kloos W.E., George C.G., Hollis R.J., Jones R.N., Pfaller M.A. 1999. Methicillin-resistant Staphylococcus aureus outbreak in a veterinary teaching hospital: potential human-to-animal transmission. J. Clin. Microbiol. 37: 1459–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shanmuganathan V.A., Armstrong M., Buller A., Tullo A.B. 2005. External ocular infections due to methicillin-resistant Staphylococcus aureus (MRSA). Eye (Lond.) 19: 284–291. [DOI] [PubMed] [Google Scholar]
- 16.Sotozono C., Fukuda M., Ohishi M., Yano K., Origasa H., Saiki Y., Shimomura Y., Kinoshita S. 2013. Vancomycin Ophthalmic Ointment 1% for methicillin-resistant Staphylococcus aureus or methicillin-resistant Staphylococcus epidermidis infections: a case series. BMJ Open 3: e001206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tajima K., Sinjyo A., Ito T., Noda Y., Goto H., Ito N. 2013. Methicillin-resistant Staphylococcus aureus keratitis in a dog. Vet. Ophthalmol. 16: 240–243. [DOI] [PubMed] [Google Scholar]
- 18.Weese J.S., Archambault M., Willey B.M., Hearn P., Kreiswirth B.N., Said-Salim B., McGeer A., Likhoshvay Y., Prescott J.F., Low D.E. 2005. Methicillin-resistant Staphylococcus aureus in horses and horse personnel, 2000–2002. Emerg. Infect. Dis. 11: 430–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yanagihara K., Araki N., Watanabe S., Kinebuchi T., Kaku M., Maesaki S., Yamaguchi K., Matsumoto T., Mikamo H., Takesue Y., Kadota J., Fujita J., Iwatsuki K., Hino H., Kaneko T., Asagoe K., Ikeda M., Yasuoka A., Kohno S. 2012. Antimicrobial susceptibility and molecular characteristics of 857 methicillin-resistant Staphylococcus aureus isolates from 16 medical centers in Japan (2008–2009): nationwide survey of community-acquired and nosocomial MRSA. Diagn. Microbiol. Infect. Dis. 72: 253–257. [DOI] [PubMed] [Google Scholar]


