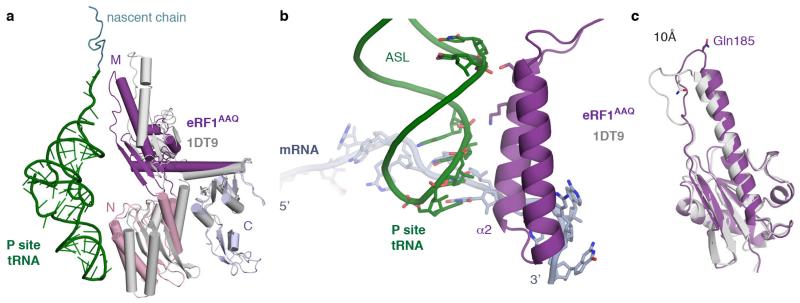
Extended Data Figure 4. eRF1AAQ interactions within the termination complex.
a, Comparison of ribosome-bound eRF1AAQ (coloured by domain) with the crystal structure of eRF1 (PDB ID: 1DT9, grey) superposed on the C domain. Both the N and M domains of eRF1 rotate upon stop codon recognition on the ribosome. The P-site tRNA (green) and nascent chain (teal) are shown for orientation. b, Interaction of helix α2 of the N domain of eRF1AAQ (purple) with the anticodon stem loop (ASL) of the P-site tRNA (green). c, Superposition of the eRF1AAQ M domain (purple) with the eRF1 crystal structure (PDB ID: 1DT9) showing a 10 Å movement of the GGQ-loop to accommodate within the peptidyl transferase centre.