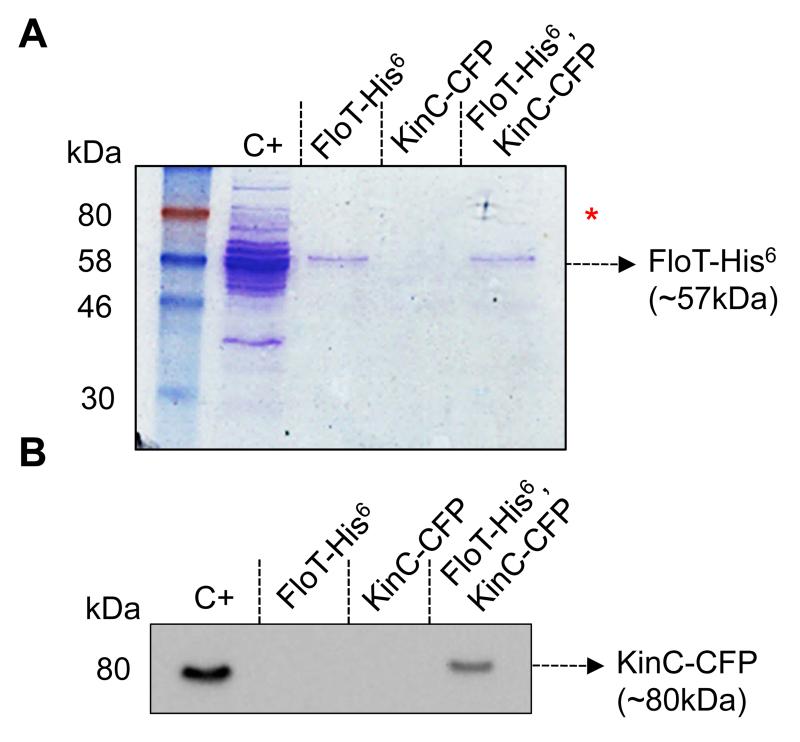
Figure 2. KinC physically interacts with FloT in B. subtilis membranes.
(A) Coomassie stained SDS-PAGE of the distinct pulled down protein samples. Positive control (C+) is the wild-type membrane fraction. The elution fraction from the FloT-His6 single-labeled strain was loaded into FloT-His6 lane. The elution fraction from the KinC-CFP single-labeled strain was loaded into KinC-CFP lane. Lane FloT-His6, KinC-CFP is the elution fraction from the FloT-His6, KinC-CFP double-labeled strain. The arrow indicates the presence of a band with the size predicted for FloT-His6. The red asterisk indicates the presence of extra protein bands in C+ and FloT-His6, KinC-CFP lanes with the size predicted for KinC-CFP. (B) Western blot assay using polyclonal antibodies against CFP to detect the presence of the KinC-CFP translational fusion in protein samples pulled down with the FloT-His6 translational fusion. The arrow indicates the presence of a band with the size predicted for KinC-CFP. Positive control (C+) is the wild-type membrane fraction. Lane KinC-CFP is the elution fraction from a nickel-charged column loaded with a sample of the membrane fraction from the KinC-CFP single-labeled strain. In the absence of FloT-His6, KinC-CFP does not bind to the column. Lane FloT-His6 is the elution fraction from the membrane fraction of a FloT-His6 single-labeled strain. No signal is detected in the absence of KinC-CFP. Lane FloT-His6, KinC-CFP is the elution fraction from the membrane fraction of a FloT-His6, KinC-CFP double-labeled strain. This lane shows a band with a molecular weight attributable to KinC-CFP, according to the C+ lane.