Fig. 1.
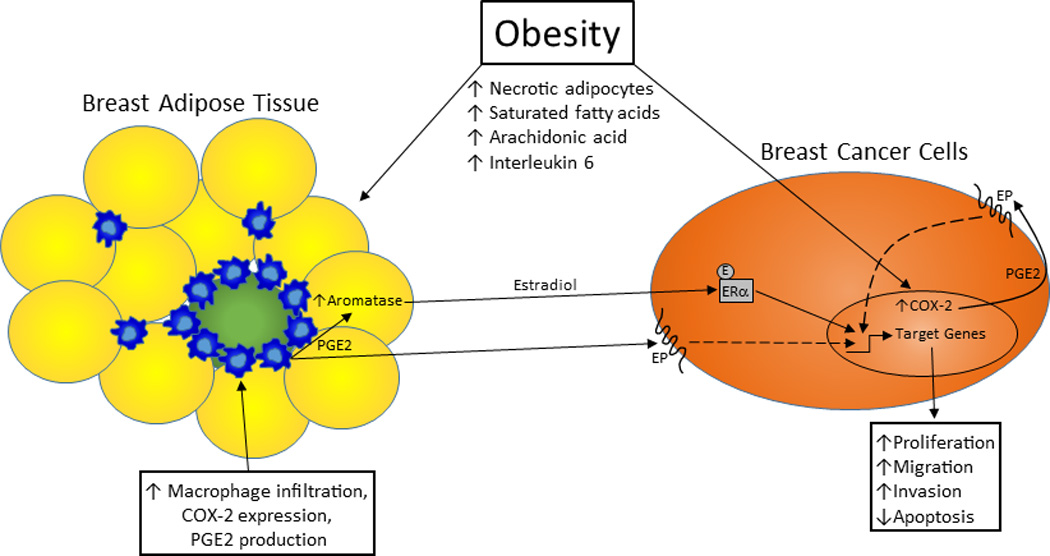
Obesity, COX-2 expression, and breast cancer progression. Obesity stimulates local COX-2 expression in the breast through multiple mechanisms, leading to increased PGE2 production that directly and indirectly promotes breast cancer progression. With the increase in adipocyte necrosis that typically accompanies obesity, there is a rise in macrophage infiltration into the breast adipose tissue. COX-2 expression in these macrophages is enhanced by an obesity-associated elevation in circulating saturated fatty acid levels, which are the result of a higher rate of lipolysis. In addition, circulating interleukin 6 levels are typically elevated with obesity, and this may induce greater breast cancer cell COX-2 expression. PGE2 production by both cell types may also be increased with obesity by higher serum arachidonic acid levels. This PGE2 can directly stimulate migration and invasion and inhibit apoptosis in breast cancer cells. It can also indirectly affect breast cancer cell proliferation, migration, and apoptosis via its induction of pre-adipocyte aromatase expression and estradiol production. COX-2: cyclooxygenase-2; E: estradiol; EP: prostaglandin receptor; ERα: estrogen receptor alpha; PGE2: prostaglandin E2
