Abstract
Xerophilic fungal species of the genus Aspergillus are economically highly relevant due to their ability to grow on low water activity substrates causing spoilage of stored goods and animal feeds. These fungi can synthesize a variety of secondary metabolites, many of which show animal toxicity, creating a health risk for food production animals and to humans as final consumers, respectively. Animal feeds used for rabbit, chinchilla and rainbow trout production in Argentina were analysed for the presence of xerophilic Aspergillus section Aspergillus species. High isolation frequencies (>60%) were detected in all the studied rabbit and chinchilla feeds, while the rainbow trout feeds showed lower fungal charge (25%). These section Aspergillus contaminations comprised predominantly five taxa. Twenty isolates were subjected to taxonomic characterization using both ascospore SEM micromorphology and two independent DNA loci sequencing. The secondary metabolite profiles of the isolates were determined qualitatively by HPLC-MS. All the isolates produced neoechinulin A, 17 isolates were positive for cladosporin and echinulin, and 18 were positive for neoechinulin B. Physcion and preechinulin were detected in a minor proportion of the isolates. This is the first report describing the detailed species composition and the secondary metabolite profiles of Aspergillus section Aspergillus contaminating animal feeds.
Keywords: animal feed spoilage, xerophilic species, teleomorphs, mycotoxins, scanning electron microscopy
1. Introduction
Fungal contamination of foods and feeds causes negative effects on the quality of the products mainly reducing their nutritional and organoleptic properties and lead consequently to important annual economic losses worldwide [1,2,3,4]. Moreover, fungi are capable of elaborating a wide range of secondary metabolites many of which have been shown to be highly toxic. Therefore, fungal contamination creates a serious health threat for animals as well as for humans. Aspergillus and its teleomorphs are important mycotoxin producers and these fungi constitute an important contaminant of cereals and feedstuffs [5,6,7,8]. Especially, Aspergillus species of the section Aspergillus—members of the teleomorphic genus Eurotium in the previous nomenclature [8]—have been reported to be able to contaminate a variety of biological materials of low water activity. These include stored grains and cereals [9], dried salted fish [10], bakery products [11], mixed feeds and raw materials [5], cultural assets and old books [12,13], and even human corpses [14]. Aspergillus section Aspergillus species have also been isolated from hypersaline waters of salterns [7], air samples near buildings [15] and from the Dead Sea [16].
Section Aspergillus species are generally considered benign fungi for human health and they are used in oriental food fermentation processes as starter cultures [8]. Species of this section have been isolated and also new species described from natural fermentation products, such as meju, a dried fermented soybean paste typically consumed in Korea [17,18,19]. Although generally considered free of mycotoxins, some of the secondary metabolites produced by the section Aspergillus fungi are known to show antioxidative, antibacterial, antifungal and antiprotozoal activities [20,21,22]. Some of these compounds, such as echinulin, physcion and flavoglaucin, even though not considered mycotoxins sensu stricto, do show toxicity to animals [23,24,25,26]. In addition, the production of more potent mycotoxins such as aflatoxins, gliotoxin, sterigmatocystin and ochratoxin, by the Aspergillus section Aspergillus species is controversial [9,27,28,29,30,31,32,33]. Several published reports of such mycotoxic potential exist, but these studies have been criticized for lacking further confirming studies using taxonomically properly characterized fungal isolates, or no repetitive mycotoxin detection has been achieved [9,27,28,29,30,31,32,33]. Therefore, the true toxicogenic capacity of Aspergillus section Aspergillus requires revision. In addition, the growth of xerophilic fungi, like the section Aspergillus, results in release of metabolic water. This increases the water activity of the contaminated materials in time. Such change in the physico-chemical properties of the substrates can permit the growth of other less xerophilic and highly toxicogenic fungi, such as Alternaria, Penicillium and Fusarium, and consequently result in further contamination of foods and feeds with even more potent mycotoxins.
The mycotoxin-contaminated animal feeds do not have potentially adverse effects only on animal health and productivity but they can cause further secondary contaminations of human consumers via eggs, meat, or milk [34,35]. Aspergillus section Aspergillus teleomorphs have been isolated from rabbit and chinchilla feeds as well as from poultry feeds with very high frequencies in Argentina [36,37]. More importantly, these studies have demonstrated that the given animal feeds were also contaminated with various potent mycotoxins (aflatoxins, deoxynivalenol, fumonisins, ochratoxin A, T2-toxin and zearalenone). Even though the detected toxin levels were lower than the regulation limits established, these contaminations with multiple mycotoxins were simultaneous and affected up to 80%–100% of the studied samples. Because the section Aspergillus fungi can produce multiple secondary metabolites with adverse effects on animal health and also reports of even more hazardous mycotoxins production exist, these high contamination levels are suggesting that both a direct and an indirect risk for animal and human health could be linked to the use of these contaminated animal feeds. This makes the further studies on the degree of xerophilic fungal contamination, the detailed species composition identification and especially, the toxicological characterization of the isolates obtained from animal feeds very valuable. Access to such information would help to evaluate the true mycotoxicological risk generated by the use of feeds in animal production in Argentina. Moreover, fungal strains isolated from different matrices, habitats and geographical origins are expected to have different metabolite profiles and toxicogenic potentials [38]. Therefore, local information is needed as the results obtained from animal feeds used in other geographical areas cannot be directly extrapolated.
The aim of the work presented here was to study in detail the degree of xerophilic fungal contamination in variable commercial and non-commercial animal feedstuff and primary raw materials destined to rabbit, chinchilla, and rainbow trout production in Argentina. The Aspergillus section Aspergillus species composition was thoroughly characterized and the production of the secondary metabolites was profiled in 20 isolates belonging to the dominant feedstuff contaminating section Aspergillus species. Especially, the evaluation of the secondary metabolite production capacity was expected to clarify the currently controversial position of section Aspergillus species as a toxin free taxon.
The Aspergillus section Aspergillus isolates obtained in this study and the previous bibliographic data were treated according to the current nomenclature rules of the Code of Botanical Nomenclature for Algae, Fungi and Plants [39] and the International Commission on Penicillium and Aspergillus (ICPA) [40]. The isolated teleomorphic fungal states are referred by their genus Aspergillus species names. This recently proposed change in the fungal nomenclature has also faced criticism as the introduction of the teleomorphic genus Eurotium into the genus Aspergillus, with the consequent changes of the species nomenclature, is feared to cause widespread confusion [41]. To avoid any confusion to the readers, the five Aspergillus section Aspergillus species most relevant to this study and supported by the recent taxon revision by Hubka and colleagues [42] are presented in Table 1, together with their corresponding former genus Eurotium names.
Table 1.
The actual nomenclature of five Aspergillus section Aspergillus species. The taxa in the teleomophic genus Eurotium have been transferred to the genus Aspergillus, according to the one-species-one name principle, and the Eurotium names should thus not be used anymore. The teleomorph of A. proliferans was described after this nomenclature change [42].
| Previous Nomenclature | Current Nomenclature |
|---|---|
| Eurotium amstelodami | Aspergillus montevidensis |
| E. chevalieri | A. chevalieri |
| E. herbariorum | A. glaucus |
| E. repens | A. pseudoglaucus |
| E. rubrum | A. ruber |
| --- | A. proliferans |
2. Results
2.1. Taxonomic Identification of Section Aspergillus Species Based on Growth and Ascospore Characteristics
The presence of xerophilic fungi of Aspergillus section Aspergillus was analysed in commercial and non-commercial producer assembled formulations of rabbit, chinchilla, and rainbow trout feeds used in animal production in Argentina. The fungal contamination of animal feeds can happen both during the pre- and post-fabrication phases. Neither do all the producers in the region use commercial animal feed formulations for animal feeding. Therefore, the presence of xerophilic fungi was also tested in a wide variety of primary raw materials used either for direct feeding or for preparation of homemade animal feed mixes by the producers (alfalfa pellets, wheat, corn and soybean derivatives, and bone and meat flour).
The initial isolation of section Aspergillus species was carried out on DG18 medium, a standard selective medium used for enumeration and isolation of xerophilic fungi from dry and semi-dry foods and feeds. Twenty-one samples of rabbit feeds, 25 samples of chinchilla feeds, 28 samples of rainbow trout feeds and one sample of each of the nine raw material types were analysed for their fungal charge leading to isolation of altogether 522 putative section Aspergillus isolates. The morphological species level identification was done according to the taxonomic key of Pitt and Hocking [8], focused on identification of five teleomorphic species dominant as contaminants of low water activity substrates worldwide. This identification resulted, in the first place, in the detection of four section Aspergillus species among the isolates: A. montevidensis, A. chevalieri, A. pseudoglaucus and A. ruber. Only a minor number of isolates could not be identified using the given taxonomic key. The total isolation frequency (Fr%) of Aspergillus section Aspergillus spp. and the Fr% of the four identified species isolated from the different animal feeds and the primary raw materials are presented in Table 2. Elevated total isolation frequencies (>60%) were obtained from both the rabbit and chinchilla feeds while the fungal charge of the studied rainbow trout feeds was somewhat lower (25%). The four identified Aspergillus species were present both in rabbit and chinchilla feeds. In the case of rainbow trout feeds, only A. pseudoglaucus and A. ruber were initially detected. However, further growth morphological analyses suggested that all the isolates initially identified as A. ruber from the trout feeds were in fact A. proliferans and that this fifth species was also present in the rabbit and chinchilla feeds analysed (see below). Among the primary raw materials, pelleted alfalfa, wheat bran, wheat millrun, pelleted soy and corn seeds all tested positive for section Aspergillus fungi. No isolates were obtained from meat and bone flower, inactivated soy or commercially produced whole grain and milled corn.
Table 2.
Total isolation frequencies (Fr%) of Aspergillus section Aspergillus spp. and those of the four identified species from the animal feeds and the primary raw materials analysed. The Fr% of the unidentified section Aspergillus spp. includes isolates which could not be identified with certainty based on their growth morphological characteristics according to the identification key of Pitt and Hocking [8]. The proportion of A. proliferans among A. ruber isolates was estimated based on their restricted growth on CY20S [42].
| Animal Feed/ Raw Material | Fr% | |||||
|---|---|---|---|---|---|---|
| Total | A. montevidensis | A. chevalieri | A. pseudoglaucus | A. ruber (A. proliferans) | Unidentified Aspergillus spp. | |
| Rabbit feed * | 61.9 | 19.1 | 23.8 | 33.4 | 14.3 (1/7) | 4.8 |
| Chinchilla feed ** | 60 | 44 | 12 | 12 | 16 (5/7) | 12 |
| Rainbow trout feed *** | 25 | ND | ND | 17.9 | 14.3 (7/7) | 3.6 |
| Pelleted alfalfa (p) | 100 | 100 | ND | ND | 100 + | ND |
| Wheat bran (p) | 100 | ND | 100 | ND | ND | ND |
| Wheat millrun (c) | 100 | 100 | ND | ND | ND | ND |
| Pelleted soybeans (p) | 100 | ND | 100 | 100+ | ND | DN |
| Corn seeds (p) | 100 | ND | ND | ND | ND | 100 |
* data based on 21 analysed samples presenting three different feed formulations, ** data based on 25 analysed samples presenting three different feed formulations, *** data based on 28 analysed samples presenting two different feed formulations. One sample of each raw material type was analysed. (p) producer’s own preparation, (c) commercial product. ND, not detected. + The initial isolate identification was corrected after SEM and DNA analyses.
Three species of section Aspergillus; A. ruber, A. proliferans and A. glaucus, are known to show very similar colony morphology on CYA, MEA and CY20S media. In addition, their general ascospore characteristics are relatively similar and these species can therefore easily be confused with each other. Due to their close resemblance, some authors have treated A. ruber and A. glaucus as synonyms or A. glaucus and A. proliferans as a single species [43,44,45,46]. Recent taxonomic analyses using both micro- and macromorphological growth characteristics, physiological data, DNA fingerprinting and four independent DNA loci sequences, however, strongly support the independent evolutionary identities of these three species [42]. This study also described the teleomorph of A. proliferans, the only species in the section Aspergillus without a link between its ana- and teleomorphic states.
According to the taxonomic key of Hubka et al. [42] A. glaucus can be distinguished from the two other species by its predominantly larger ascopores (6–7.5(8.5) µm), while A. ruber is able to grow on M60Y medium at 37 °C, a characteristic not shared by A. proliferans. Also, the growth of A. proliferans is significantly more restricted on CY20S medium at 25 °C after seven days (<30 mm) than the growth of A. ruber or A. glaucus (>30 mm). Unfortunately, the information of this different growing capacity on M60Y medium as a fundamental tool for discrimination between A. ruber and A. proliferans was not available during the experimental set up of the present study. Nevertheless, the evaluation of the obtained A. ruber isolates for their ascospore sizes suggested total exclusion of A. glaucus. Even though relative high size variation could be detected even within individual isolates, the mean ascospore sizes were closer to 5 than 6 µm and no isolates with larger ascopores, characteristic for A. glaucus, were detected. On the other hand, the growth responses on CY20S medium were indicating a heterogeneous nature for the initial A. ruber isolate collection. These could be divided into two marked growth response groups; one with maximum up to 30 mm diameter colonies (i.e., A. proliferans) and one with significantly vigorous colony growth (>40–50 mm), characteristic for A. ruber. This suggested the presence of A. proliferans as a fifth section Aspergillus species isolated from the starter materials. Later, the DNA sequence data further confirmed this growth morphological identification of A. proliferans (see below) allowing a rough estimation of the presence of A. proliferans among the isolates initially identified as A. ruber (Table 2).
The taxonomic characterization was continued with 20 isolates including representatives of four growth morphologically identified species, A. montevidensis, A. chevalieri, A. pseudoglaucus and A. ruber. This set of isolates consisted of five representatives of the first three section Aspergillus species and four of A. ruber. In addition, one putative isolate of A. proliferans, identified by its restrictive growth response on CY20S medium, was included for confirming this growth morphological detection of the species. The isolates originated from all three types of animal feed mixes studied and from pelleted soy and alfalfa used as primary raw materials. The 20 isolates were subjected to further electron microscopic analysis, DNA level taxonomic characterization and secondary metabolite profiling.
2.2. Scanning Electron Microscopy (SEM)
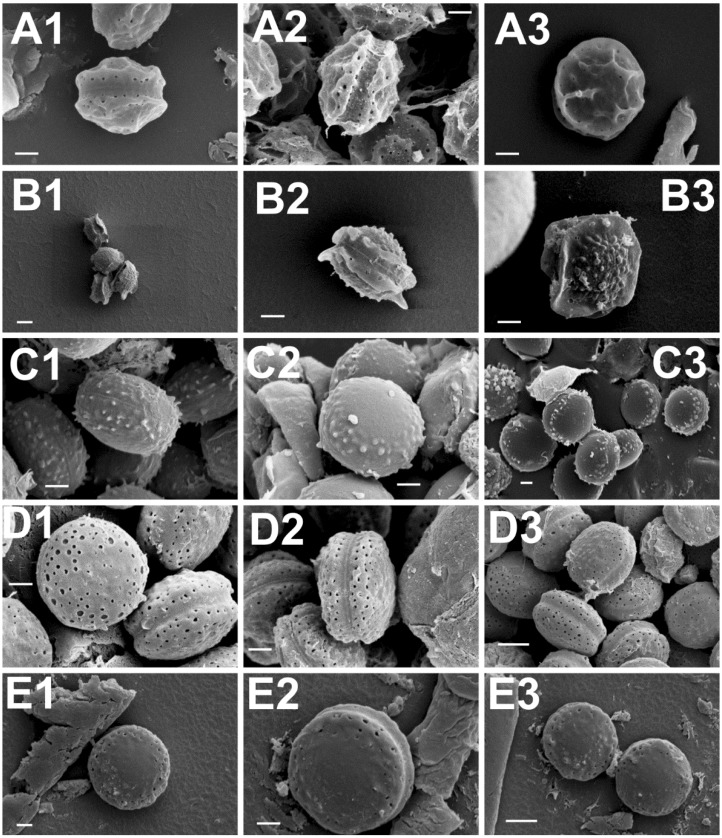
Scanning electron microscopy (SEM) has traditionally been used as a tool for species classification in the section Aspergillus. Especially ascospore size, surface ornamentation and features of the equatorial region have offered characteristics of taxonomic value for species differentiation. To further confirm the species identity of the selected isolates, their ascospore micromorphology was analysed by SEM. The results were concordant with the growth morphological species identification and confirmed the isolates as representatives of the respective four Aspergillus section Aspergillus species. The ascospore characteristics did not significantly differ from those already described [8,47]. A set of representative SEM photos are shown in Figure 1 and a summary of the ascospores characteristics is presented in Table 3. Two isolates, originally identified based on the growth characters and light microcopy observation of the ascospores as A. montevidensis, were re-identified as A. chevalieri by SEM. In addition, one isolate originally considered as A. chevalieri was re-identified as A. ruber. These changes in the isolate species identity were further supported by DNA analyses (see below) and demonstrates the value of SEM as a complementary tool for species identification in the section Aspergillus. It is however noteworthy that the ascospores of A. proliferans and A. ruber have highly similar size and micromorphology and therefore SEM does not offer solid separation of these two species. Moreover, some strains of A. ruber are reported to show atypical ascospore morphology making spore size and surface ornamentation very weak identification characteristics for A. ruber and A. proliferans [42]. Our SEM study resulted in detection of slightly bigger and smoother surfaced ascospores in the case of the studied A. proliferans isolate; otherwise, the general morphological characteristics and the size of the ascopores between A. proliferans and A. ruber were highly similar. These results confirm the weak taxonomic resolution of SEM in separating A. ruber from A. proliferans, a goal that, besides some growth characteristics, can solidly be reached by DNA analyses.
Figure 1.
Scanning electron microscopy photos of ascospores of A. montevidensis (A, 1–3); A. chevalieri (B, 1–3); A. pseudoglaucus (C, 1–3); A. ruber (D, 1–3) and A. proliferans (E, 1–3). Scale bars: A(1–3)-B(2,3)-C(1–3)-D(1–3)-E(1,3) = 1 µm; B1, E2 = 2 µm.
Table 3.
Ascospore sizes (µm) and the micromorphological characteristics of the five Aspergillus section Aspergillus species isolated from feedstuffs.
| Species | Ascospore Measurements (µm) and Ornamentation | ||||
|---|---|---|---|---|---|
| Size | Surface | Furrow | Pores | Ridges | |
| A. montevidensis | 4.2–4.5 | reticulated | pronounced | 0.1 | short |
| A. chevalieri | 3.8–4.6 | small peaks | shallow | 0.1–0.2 | long, wavy |
| A. pseudoglaucus | 4.2–4.6 | small peaks | minimum | absent | absent |
| A. ruber | 4.6–5.8 | protuberances | pronounced | 0.1–0.2 | absent |
| A. proliferans | 5.2–5.3 | smooth | pronounced | 0.1–0.3 | absent |
2.3. Taxonomic Identification Based on DNA Analyses
The final confirmation of species identity of the 20 isolates was obtained through PCR amplification and sequencing of the nuclear ribosomal ITS1-5.8SrDNA-ITS2 region and beta-tubulin gene fragment. The recent combined phylogenetic analysis of Hubka and colleagues [42], based on three independent protein encoding DNA loci (beta-tubulin, calmodulin and RNA polymerase domain 2), has postulated recognition of altogether 17 Aspergillus section Aspergillus species. Fourteen of them are located within three major clades, each of which with strongly supported terminal clades corresponding to independent species of the section. Aspergillus glaucus clade includes closely related species of A. glaucus and A. proliferans, A. niveoglaucus, A. brunneus and A. neocarnoyi while A. ruber clade consists of A. ruber, A. pseudoglaucus, A. appendiculatus and A. tonophilus. The A. chevalieri clade is formed by A. chevalieri, A. montevidensis, A. intermedius, A. cristatus and A. costiformis. The only species located outside of these three major clades are A. cibarius, A. xerophilus and A. leucocarpus [42]. Posterior to Hubka’s analyses also two new species, A. cumulatus and A. osmophilus have been described from rice straw used in meju fermentation in Korea [19] and from contaminated cereals in Iran [48]. Then, a study conducted by Visagie et al. [49] described the new species A. sloanii on Aspergillus section Aspergillus isolated from indoor house dust in United Kingdom. The morphological characteristics and DNA data indicates that these are true new evolutionary species reaching a total of 20 species in Aspergillus section Aspergillus.
The phylogenetic analyses of the 20 studied isolates were conducted using the type-strain reference sequences of altogether 18 currently described section Aspergillus species. The accession numbers of the studied isolates are presented in Table 4. The species, strain numbers and sequence of the type-strain reference and more detailed information of the previous nomenclature are available in Supplementary Materials (Table A1 and Table A2, respectively). In the case of the four species, A. montevidensis, A. chevalieri, A. pseudoglaucus and A. ruber, not only the type-strain sequences but also non-type strains were included for increasing the sequence diversity. Aspergillus osmophilus was excluded from the analyses due to its very large ascospore size (8.5–10 µm), a characteristic which was not detected among our 20 isolates. At the DNA marker level, this species is most related to A. xerophilus and does not form part of the three major terminal multispecies clades of the section [48]. The proposed novel species, A. cumulatus, was however included as its ascospore characteristics overlap with the studied isolates [19].
Table 4.
ITS and beta-tubulin sequences of the isolates under study.
| Species Name | Isolate | GenBank Accession Number | |
|---|---|---|---|
| ITS | Beta Tubulin | ||
| A. montevidensis | 2 | KT373923 | KT373942 |
| A. montevidensis | 4 | KT373925 | KT373944 |
| A. montevidensis | 20 | KT373941 | KT373960 |
| A. chevalieri | 1 | KT373921 | KT373922 |
| A. chevalieri | 3 | KT373924 | KT373943 |
| A. chevalieri | 5 | KT373926 | KT373945 |
| A. chevalieri | 6 | KT373927 | KT373946 |
| A. chevalieri | 7 | KT373928 | KT373947 |
| A. chevalieri | 8 | KT373929 | KT373948 |
| A. proliferans | 16 | KT373937 | KT373956 |
| A. pseudoglaucus | 10 | KT373931 | KT373950 |
| A. pseudoglaucus | 11 | KT373932 | KT373951 |
| A. pseudoglaucus | 12 | KT373933 | KT373952 |
| A. pseudoglaucus | 13 | KT373934 | KT373953 |
| A. pseudoglaucus | 14 | KT373935 | KT373954 |
| A. pseudoglaucus | 15 | KT373936 | KT373955 |
| A. ruber | 9 | KT373930 | KT373949 |
| A. ruber | 17 | KT373938 | KT373957 |
| A. ruber | 18 | KT373939 | KT373958 |
| A. ruber | 19 | KT373940 | KT373959 |
In order to confirm the presence of A. proliferans among the studied isolates, special attention was paid to this taxon. The recently described A. proliferans teleomorph is from many growth morphological characteristics highly similar to A. ruber, while at the DNA maker level it is very close to its sister species, A. glaucus [42]. As being frequently misidentified in the past, the true global species abundance and the intraspecies sequence variation of A. proliferans is not well known yet. Neither has the teleomorph of this species been described in Argentina before. In order to increase the diversity of the geographical origin of the isolates, not only the A. proliferans type-strain, isolated from United Kingdom, but also non-type isolates from United States and Czech Republic and two fungal isolates, described as novel A. proliferans teleomorphs from Iran [48], were included in the analyses.
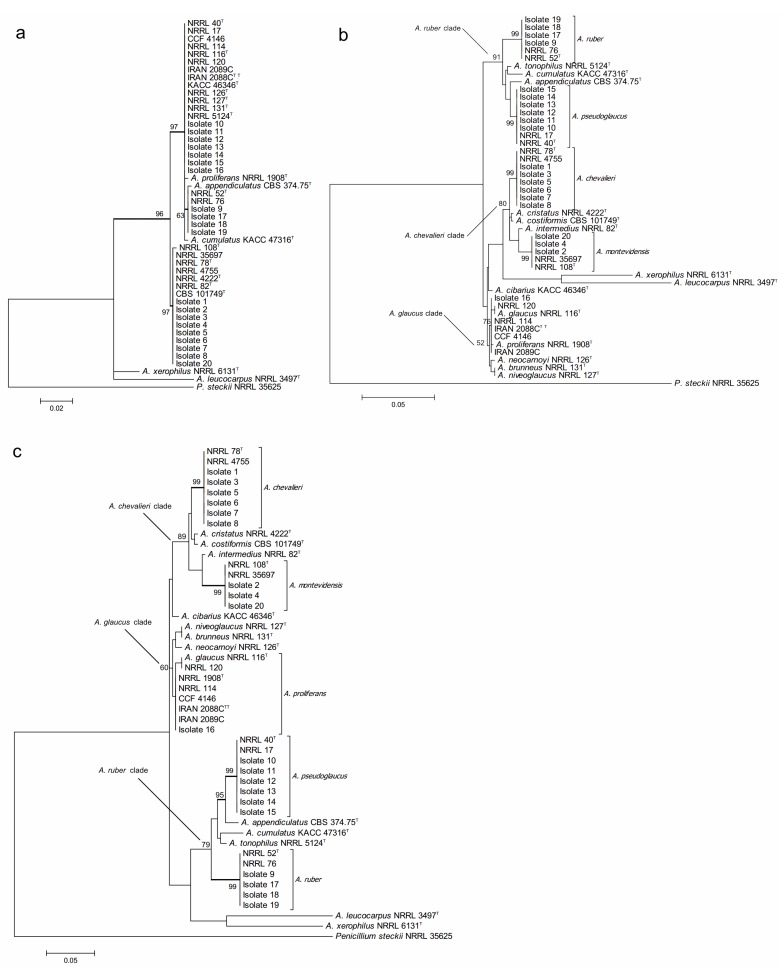
The maximum likelihood analysis based on the ITS sequences (Figure 2a) resulted in very weak resolution of the Aspergillus section Aspergillus at species level. All the 20 studied isolates located within two major multi-species terminal clades with strong bootstrap support but none of them could be identified at species level. One of the clades included both the 11 studied isolates and all the species of A. glaucus and A. ruber clades, and A. cibarius. Within this multispecies clade, four of the studied isolates formed part of a moderately supported internal subclade. However, this subclade was neither conspecific nor included both A. ruber and A. appendiculatus. The second major multispecies clade consisted of the rest of the nine studied isolates and the species known to belong to the A. chevalieri clade of the section.
Figure 2.
Phylogenetic trees generated by Maximum Likelihood analysis showing the relationship of 18 type strains of Aspergillus section Aspergillus species and the 20 isolates obtained from animal feeds and primary raw materials. Three based on (a) the ITS region; (b) the beta-tubulin gene fragment; and (c) the concatenated analysis of both. Penicillium steckii (NRRL 35625) was used as outgroup. The thickened lines represent lineages with >90% bootstrap values. The Bootstrap analyses were performed with 1000 replications. T Ex-type strain TT Ex-teleotype strain.
On the other hand, the phylogenetic analysis based on the beta-tubulin encoding gene fragment resulted in discrimination between altogether 17 of the 18 Aspergillus section Aspergillus species included in the study. Only A. niveoglaucus and A. brunneus could not be separated from each other with this DNA marker. The study also successfully confirmed the species identity of all the studied isolates (Figure 2b). The growth morphological species identification with the additional SEM data was confirmed and the studied isolates were identified as three isolates of Aspergillus montevidesis, six of A. chevalieri, six of A. pseudoglaucus, four of A. ruber and one as A. proliferans. The morphological identification of A. proliferans from A. ruber based on its restricted growth on CY20S medium was confirmed as the given isolate (isolate 16) located together with the four other A. proliferans sequences used in the analysis. The species identity of only one of the isolates, considered A. ruber before DNA analysis changed to A. pseudoglaucus.
The sequences of the 20 studied isolates were in all the cases 100% identical to GenBank type-strain reference sequences. The three characteristic multispecies clades of the section (A. chevalieri, A. glaucus and A. ruber clades) were clearly detectable and all the terminal species clades with studied isolate sequences were strongly supported having bootstrap values higher than 80.
A further concatenated phylogenetic study was run joining the ITS and b-tubulin sequences data (Figure 2c). As expected, this analysis did not change the species identity of the studied isolates but it did slightly modify the bootstrap support of the three major clades and some of the terminal clades as well. In most of the cases, the support of the nodes was reduced. Joining the two DNA loci data did not result in separation between A. niveoglaucus and A. brunneus either, demonstrating that the maximum species level discrimination for Aspergillus section Aspergillus was reached already with one protein encoding DNA locus, the b-tubulin fragment.
2.4. Secondary Metabolite Profiles
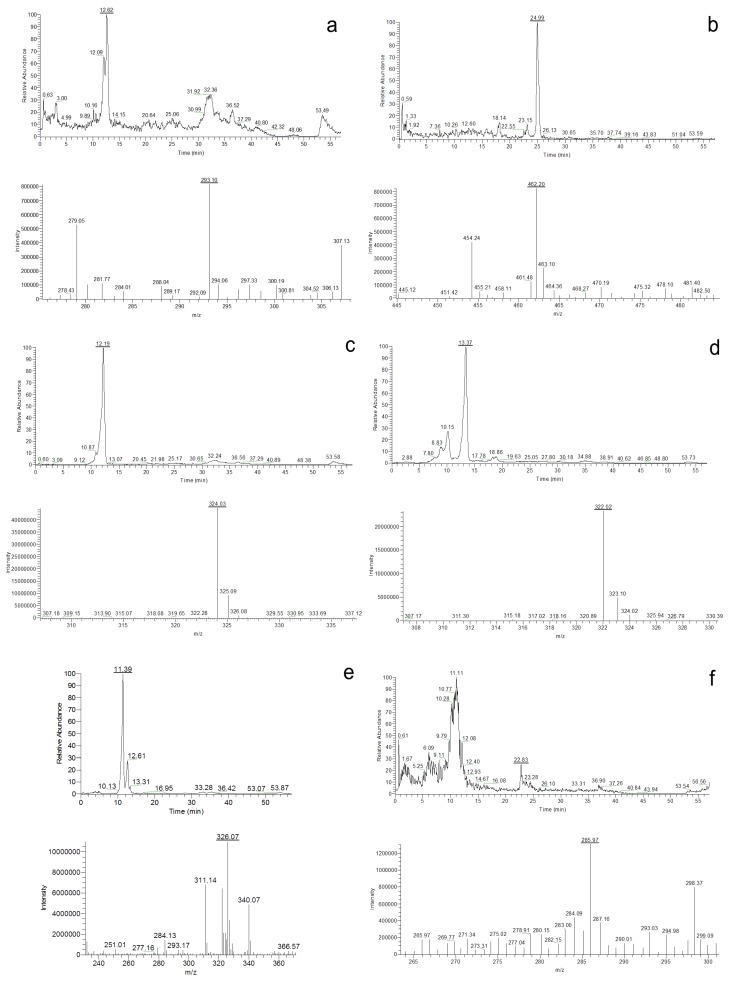
Once the species identity was comprehensively established for the 20 isolates, their secondary metabolite profiles were analysed. The HPLC-MS analysis showed the presence of cladosporin, echinulin, and neoechinulin A and B (Table 5). All the studied isolates, representatives of the five species of section Aspergillus, were able to produce neoechinulin A. Eighteen isolates produced neoechinulin B, and 17 isolates were positive for cladosporin and echinulin. In addition, preechinulin and physcion were also detected. The Figure 3 shows representative chromatograms and the ESI spectrum of the determined secondary metabolites. None of the species showed a fixed profile, but a moderate intraspecies variation in the secondary metabolite production was detected.
Table 5.
Secondary metabolites of the five Aspergillus section Aspergillus species isolated from feeds and feedstuffs (ND: Not Detected).
| Species | Secondary Metabolite | |||||
|---|---|---|---|---|---|---|
| Cladosporin | Echinulin | Neoechinulin A | Neoechinulin B | Preechinulin | Physcion | |
| A. montevidensis | + | + | + | + | + | ND |
| A. montevidensis | + | + | + | + | ND | ND |
| A. montevidensis | + | + | + | + | ND | ND |
| A. chevalieri | + | ND | + | + | + | ND |
| A. chevalieri | + | + | + | + | ND | + |
| A. chevalieri | + | + | + | + | ND | + |
| A. chevalieri | + | + | + | + | ND | ND |
| A. chevalieri | + | + | + | + | ND | ND |
| A. chevalieri | + | + | + | + | ND | ND |
| A. pseudoglaucus | + | + | + | + | ND | ND |
| A. pseudoglaucus | + | + | + | + | ND | ND |
| A. pseudoglaucus | + | + | + | + | ND | ND |
| A. pseudoglaucus | ND | + | + | + | ND | ND |
| A. pseudoglaucus | + | ND | + | ND | ND | ND |
| A. pseudoglaucus | + | ND | + | + | ND | ND |
| A. ruber | ND | + | + | + | ND | ND |
| A. ruber | ND | + | + | + | ND | ND |
| A. ruber | + | + | + | + | ND | ND |
| A. ruber | + | + | + | ND | ND | ND |
| A. proliferans | + | + | + | + | ND | ND |
Figure 3.
Chromatograms and ESI spectrum of (a) Cladosporin (m/z 293); (b) Echinulin (m/z 462); (c) Neoechinulin A (m/z 324); (d) Neoechinulin B (m/z 322); (e) Preechinulin (m/z 326); (f) Physcion (m/z 285).
All three A. montevidensis isolates were able to produce cladosporin, echinulin, neoechinulin A and B while one of them was also producing preechinulin.
The culture extracts of the six A. chevalieri showed presence of cladosporin and neoechinulin A and B and in only one of the isolates echinulin was not detected. The same A. chevalieri isolate was also positive for preechinulin and two other were detected producing physcion.
From the six A. pseudoglaucus isolates, three were positive for all four secondary metabolites; cladosporin, echinulin, and neoechinulin A and B. One isolate produced both echinulin and neoechinulin A and B but did not produce cladosporin while the two other isolates were positive for cladosporin, neoechinulin A but negative for echinulin. One of these strains did however test positive for neoechinulin B production.
Four filtrate extracts of A. ruber strains were analysed. Among them, one isolate contained cladosporin, echinulin, neoechinulin A and B, another isolate was positive for cladosporin, echinulin and neoechinulin A. The remaining two isolates were positive for echinulin and neoechinulin A and B.
The Aspergillus proliferans isolate studied showed production of cladosporin, echinulin, and neoechinulin A and B.
3. Discussion
Aspergillus section Aspergillus species are found in foods and feeds at all aw levels but they are able to cause spoilage even below 0.90 aw [11]. They are also important secondary metabolite producers and, therefore, the section Aspergillus species, previously known as members of the teleomorphic genus Eurotium, are considered one of the most destructive xerophilic fungi. Very high isolation frequencies of section Aspergillus taxa have been previously reported for rabbit, chinchilla and poultry feeds in Argentina. These feeds have also tested positive for multiple very hazardous mycotoxins [36,37], suggesting that potential mycotoxicological risks could be linked to their use. Consumption of mycotoxin-contaminated feeds can provoke animal illnesses leading to unnecessary suffering and economic losses. In addition, secondary contaminations of humans as final consumers are possible via consumption of contaminated meat and other animal origin products.
Even though generally considered free of potent mycotoxins, section Aspergillus fungi are important producers of metabolites with toxic potential [6,7]. They can produce a wide variety of secondary metabolites such as echinulin, neoechinulin A, neoechinulin B, preechinulin, cladosporin, questin and physcion [7,15,50,51]. These compounds have received less attention than more potent mycotoxins, produced predominantly by other fungal species. Therefore, their presence in foods and feeds is not currently controlled or regulated in any country. However, numerous reports exist on the toxic effects of the secondary metabolites produced by section Aspergillus species on animals over the last 70 years. According to Cole and Cox [52], some Aspergillus species (A. chevalieri and A. montevidensis) should be considered mycotoxicogenic, and in these cases, echinulin has been the main toxic compound produced. Concordantly, Ali et al. [26] have reported liver and lung damages caused by echinulin in rabbits. Furthermore, strains of section Aspergillus species contaminating cereal grains have been demonstrated to be toxic for experimental animals, causing lowered weight gains in chickens, toxicity to chicken embryos, dermatoxicity in rabbits, hemorrhaging in chickens, hepatotoxicity in mice, and death of calves, rabbits and mice [53,54,55,56,57,58,59]. In addition, Vesonder et al. [59] reported that a feed refused by swine, a situation which led to decreased milk production and consequently to piglets death, was contaminated with echinulin (8 µg/g) and contained high propagule density of A. montevidensis and A. chevalieri. These fungal isolates were further confirmed to be able to produce echinulin in vitro on rice or cracked corn. On the other hand, compounds isolated from A. montevidensis and A. chevalieri show different activity against malaria, bacteria and cancer cell lines [21,60], metabolites isolated from A. cristatum have inhibitory activity on tumour cell lines [61], alkaloids produced by A. ruber show anti-oxidant activity [62], and secondary metabolites isolated from A. pseudoglaucus (physcion and echinuilin) are cytotoxic to sex cells of the sea urchin Strongylocentrotus [63].
In the light of such broad evidence of variable toxic effects of secondary metabolites produced by the section Aspergillus, these fungal species can hardly be simply considered benign to animal or human health without further investigation. Variation in the toxicogenic potential between different species and even different isolates are expected to exist. Therefore, in order to evaluate the potential mycotoxicogenical risk linked to section Aspergillus contaminations, further studies on the general fungal abundance, species composition and especially on the secondary metabolite profiles of different species and isolates are needed. Such data are not currently available for section Aspergillus fungi isolated from foods or animal feeds.
With these means, we have conducted a detailed evaluation of the xerophilic mycobiota present in various commercial and non-commercial feed formulations and primary raw materials used for rabbit, chinchilla and rainbow trout production in Argentina. The isolation trials and the growth morphological species identification confirmed the previous reports [36] of the high contamination frequencies of the rabbit and chinchilla feeds by Aspergillus section Aspergillus taxa (>60% of the studied samples), while the rainbow trout feeds showed somewhat lower fungal charge (25%). In addition, some of the primary raw materials tested (pelleted alfalfa, soy, wheat bran, wheat millrun and corn seeds) were positive for section Aspergillus fungi. These contaminations consisted predominantly of multiple species but the specific species compositions varied both between the feeds and the primary raw material types studied. The presence of five dominant section Aspergillus species was identified both in the rabbit and chinchilla feeds (A. montevidensis, A. chevalieri, A. pseudoglaucus, A. ruber and A. proliferans). However, in chinchilla feed samples A. montevidensis was the most abundant taxon, while in rabbit feeds three species; A. montevidensis, A. chevalieri and A. pseudoglaucus were the most frequently isolated ones, led by A. pseudoglaucus. On the other hand, rainbow trout feeds showed a completely different species composition formed only by two species, A. pseudoglaucus and A. proliferans, both with quite similar isolation frequencies. These variations in species abundance and composition most probably reflect different natures and complexities of the primary raw materials used for manufacturing different animal feed formulations, even though possible impacts of post-fabrication storage conditions cannot be excluded. This conclusion was supported further by the isolation results from the primary raw materials studied. When tested positive, fungal contamination was by a single or maximum two section Aspergillus species and species compositions were clearly variable between the different types of primary raw material analysed.
The correct and solid species identification is the base of any biological study. The taxonomy and nomenclature of Aspergillus section Aspergillus species has been under revision during the last years. The transfer of the teleomorphic genus Eurotium to the anamorphic Aspergillus genus, according to the one-species-one scientific name concept of the botanical code, has led to re-evaluation of the previous data on linkage of anamorphic and teleomorphic fungal states and the species identity. Unfortunate misidentifications of culture collection deposited isolates, contradictory posterior species descriptions and the use of various synonymous species names during the years has seriously complicated this task. However, recently, DNA analyses, together with physiological and growth characters, have been used for clarifying the true taxonomic species position and to solidly link the teleomorphs with their anamorphic states. The studies of Peterson [64] and Hubka et al. [42] have profoundly revised the previous species nomenclature and the identity of taxa in the section Aspergillus. The growth morphological characters and light microscopical observation of the ascospores, traditionally used for section Aspergillus species identification, are to some extent error prone due to their subjectivity. Currently, access to vast molecular taxonomic data from type-strains of section Aspergillus species allows a fast and precise direct isolate identification or growth morphological species identity confirmations.
The taxonomic position of the 20 isolates, representatives of the five dominant feedstuff contaminant species of section Aspergillus: A. montevidensis, A. chevalieri, A. proliferans, A. pseudoglaucus and A. ruber, identified by their growth morphological characters, was confirmed further using both scanning electron microscopy (SEM) and two independent DNA loci. Scanning electron microscopy allows detailed observation and description of ascospore size and structural characteristics, such as surface ornamentation, pores and their arrangement, longitudinal groove and ridges. These have served and still do serve as important characteristics for species identification in the section Aspergillus [8,17,42,47,65,66]. However, the ascospore characteristics can also be highly similar between different species, like is the case with A. ruber and A. proliferans. In addition, a relative high intraspecific and even intraisolate variation of the ascospore characteristics can exist. Therefore, SEM as a species classification and identification tool has a supplementary value, but it is not conclusive. This was confirmed by our SEM study as it allowed to correct some erroneous growth morphological species identifications but did not offer separation between A. ruber and A. proliferans isolates.
The conclusive confirmation species identity of the isolates was reached with the phylogenetic analysis based on the beta-tubulin gene fragment. The nuclear ITS region has been established as a universal DNA barcode marker for fungi [67]. However, this marker does not necessary have a species level resolution in all fungal taxa. Specifically in the genus Aspergillus, identical ITS sequences are known to be shared between several complexes. Therefore, other general protein encoding DNA loci (such as beta-tubulin, calmodulin or RNA polymerase subunit 2) are frequently used for species identification [40,42,64,68]. The degree of ITS species resolution can also vary between different sections of Aspergillus. For example, a phylogenetic study based on ITS sequences of the Aspergillus section Circumdati resulted in identification of 18 out of 27 species [69]. In our phylogenetic analyses of 18 section Aspergillus species, the ITS did not allow species level identification for any of them. On the other hand, the analysis based on the beta-tubulin gene fragment led to species level identification for 16 out of 18 of the reference taxa and of all the studied isolates. These results are concordant with reports of beta-tubulin locus as a very potent single DNA marker for species level resolution in Aspergillus section Aspergillus [17,42,66,70].
High isolation frequencies of section Aspergillus taxa were obtained from the animal feeds analysed in the present study similar to earlier reports from Argentina [36,37]. This raises the question of the already existing or potential contamination of such feeds with multiple bioactive and toxic secondary metabolites known to be produced by the section Aspergillus fungi. In addition, the evaluation of the potential toxicological risks requires previous knowledge on the toxicogenic capacity of the section Aspergillus species contaminating feeds. No previous information exist on secondary metabolite profiles of the section Aspergillus species isolated from animal feeds in Argentina and, to our knowledge, from any other country in the world. The secondary metabolite profiling of the 20 isolates belonging to the five most predominant section Aspergillus species contaminating different animal feeds revealed that, on the one hand, the five species produce a shared common metabolite profile. All the species were able to produce, under the experimental culture conditions used, cladosporin, echinulin, and neoechinulin A and B. However, variations in the secondary metabolite profile exist between the isolates of the same species. Only in the case of A. montevidensis the three isolates tested were all positive for these four secondary metabolites. In addition, preechinulin production was detected in one A. montevidensis and A. chevalieri isolates and physcion in two A. chevalieri isolates.
Production of auroglaucins, flavoglaucins and anthraquinones, such as physcion, catenarin, questin and questinol, has been reported before for A. montevidensis, A. chevalieri, A. pseudoglaucus. A. ruber, A. glaucus and A. proliferans [7]. None of these compounds, except physcion, was detected in the studied isolates. In addition, A. montevidensis and A. ruber isolates have also been reported to produce epiheveadride [15]. We did not detect this secondary metabolite in any of the studied isolates. On the other hand, all three of our A. montevidensis isolates and two of four A. ruber isolates tested positive for cladosporin, a compound that was not detected in the given species by Slack et al. [15].
Our secondary metabolite profiling results demonstrate that the Aspergillus section Aspergillus species isolated from different matrices and geographical origins do show different toxinogenic potential. All the five main species contaminating the animal feeds under study in Argentina had a consistent but, at the same time, rather limited secondary metabolite profile, at least under the culture conditions tested. No significant variation exists between species, though minor intraspecies isolate variations were detected. However, all the isolates were capable of producing echinulin, the precise secondary metabolite demonstrated to have serious mycotoxic effects on pigs, mice, rabbits [26,52]. The highest toxinogenic potential was detected in A. chevalieri as this species, depending on the isolate, tested positive for altogether six secondary metabolites.
This is the first report on detailed taxonomic identification and secondary metabolite profiling of Aspergillus section Aspergillus fungi contaminating feeds and primary raw materials used for animal feeding in Argentina. So far, most studies have observed additive or synergistic effects between consumption of different potent mycotoxins [71,72,73]. However, the effects of chronic simultaneous consumption of sub-toxic concentrations of fungal metabolites of lower toxicogenic potential, like the ones produced by the section Aspergillus, on animal or human health are currently poorly studied. Neither it is known if these compounds show additive or synergistic effects with each other or with other more hazardous mycotoxins. Therefore, further studies are needed to determine the full toxicogenic potential of Aspergillus section Aspergillus species under variable growth conditions to assess mycotoxicological risk that these xerophilic fungi can generate for animal and human health as feed and food contaminants.
4. Experimental Section
4.1. Samples
The teleomorphs of Aspergillus section Aspergillus were isolated from animal feeds and primary raw materials destined to rabbit, chinchilla and rainbow trout production in Buenos Aires, Córdoba, La Pampa, La Rioja, Mendoza, Rio Negro and Neuquén provinces in Argentina. Three different preparations of the rabbit, three of the chinchilla and two of the rainbow trout feeds were used for the isolation assays. The raw materials analysed consisted of alfalfa pellets, wheat derivatives (wheat millrun and wheat bran), bone and meat flour, soybean derivatives (pelleted soybeans and heat-inactivated soy) and corn derivatives (corn seeds and milled corn). Both the feeds and the primary raw materials studied included commercial products and animal producer fabricated non-commercial preparations. The fungal isolates obtained were maintained on solid CY20S medium at 4 °C and preserved on 18% glycerol stocks at −20 °C.
All the representative isolates described in this study are available upon request and will be accessioned in an international culture collection abroad.
4.2. Isolation of the Xerophilic Fungi
The initial isolation of xerophilic fungi and the genus level identification of Aspergillus, section Aspergillus isolates were carried out on dichloran 18% glycerol agar, DG18 [74]. The animal feeds and primary raw materials were processed according to Greco et al. [36] the serial diluted samples were inoculated on the selection medium and incubated at 25 °C for 7 days. In total, 21 samples from three different rabbit feeds, 25 from three chinchilla feeds, 28 from two rainbow trout feeds and one sample of each from the nine primary material types were analysed. The presence of section Aspergillus fungi was evaluated based on the growth morphology characteristics and isolated for further analyses. The isolation frequency (Fr%) of Aspergillus section Aspergillus was calculated for each starting material according to González et al. [75] with some modifications:
4.3. Species Level Taxonomic Identification Based on Morphological Characters
The fungal isolates identified as members of Aspergillus section Aspergillus spp. in the initial xerophilic screen were used for further morphological species level identification according to Pitt and Hocking [8]. The isolates were inoculated in three points on CYA, MEA, GN25 and CY20S media and incubated at 5, 25 and 37 °C for 7 days. The colony characteristics were recorded after the incubation periods. The CY20S plates were incubated at 25 °C for 7 days further to allow ascospore development. The microscopic ascospore characteristics were observed and the sizes measured from lactophenol cotton blue stained samples using Nikon Eclipse E200 light microscope. The ability of the strains to grow on CY20S at 37 °C for 7 days was also evaluated [42]. The isolation frequencies (Fr%) of the five identified section Aspergillus species (A. montevidensis, A. chevalieri, A. pseudoglaucus, A. ruber and A. proliferans) were calculated for each starting material according to González et al. [75] as follows:
| (2) |
4.4. Scanning Electron Microscopy (SEM)
For scanning electron microscopy analysis, the mature cleistothecia were harvested from fungal colonies grown for two weeks on CY20S and fixed in 0.1 M cacodylate buffer with 1% glutaraldehyde and 0.4% formaldehyde at room temperature [7]. The SEM service was purchased from Centro Científico Tecnológico CONICET Bahía Blanca (Argentina).
4.5. Taxonomic Identification Based on DNA Sequences
For mycelium harvest, fungi were grown for 7 days at 25 °C on solid CY20S plates. The mycelia were collected by scraping the surface of the plates and stored at −80 °C until use. The genomic DNA was extracted from 100–150 mg of mycelia manually grounded in a mortar with liquid nitrogen and the using DNeasy Plant Mini Kit (Qiagen, Intl.) according to manufacturer’s protocol. The quantification of genomic DNA was done with the fluorometer Qubit 2.0 (Life Technologies, Intl.).
The internal transcribed spacer (ITS) of nuclear ribosomal DNA was amplified using the primers ITS1 (5'-TCCGTAGGTGAACCTGCGG-3') and ITS4 (5'-TCCTCCGCTTATTGATATGC-3') [76]. The beta-tubulin gene fragment was amplified using the primers bt2a (5'-GGTAACCAAAT CGGTGCTGCTTTC-3') and bt2b (5'-ACCCTCAGTGTAGTGACCCTTGGC-3') [77]. The reaction volume used in PCR amplification was 40 µL and contained 1X of Taq buffer (Fermentas, Intl.), 1 µM of each primer (GBT Oligos, Buenos Aires), 0.2 mM of dNTPs (Fermentas, Intl.), 1.5 mM of MgCl2 (Fermentas, Intl.), 1 U of Taq DNA polymerase (Fermentas, Intl.) and 2 µL of genomic DNA template (3–15 ng/µL). The PCR amplification protocol for both primer pairs consisted of an initial denaturation step of 3 min at 94 °C, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 50 °C for 30 s, extension at 72 °C for 1 min and a final extension step at 72 °C for 5 min. The PCR reactions were performed in a T-Personal thermocycler (Biometra, GmbH, Göttingen, Germany). The amplification products were separated by electrophoresis in a 1% agarose gel, stained with ethidium bromide, visualized with a UV transilluminator and documented with a Kodak Digital Science 1D system. Sequencing of the PCR amplicons was done with ITS1 and bt2a primers at Macrogen Inc. (Seoul, Korea).
4.6. Phylogenetic Analyses
Phylogenetic analyses based on the ITS and beta-tubulin sequences were run using the MEGA 6 program package [78]. The Aspergillus section Aspergillus type-strain reference sequences of altogether 18 species used in the analyses were obtained from GenBank (http://www.ncbi.nlm.nih.gov/genbank/). The species strain numbers, and the ITS and beta-tubulin sequences with their respective GenBank accession numbers are indicated in Supplementary Materials in Table A1. (Additional information of the strains and their former taxonomic nomenclature is available at Supplementary Materials in Table A2. The sequences of Penicillium steckii, strain NRRL 35625, were used as outgroup in the analyses. The isolate nucleotide sequences were visually inspected and aligned together with the reference sequences using the Muscle method [79]. The Maximum Likelihood analyses on the ITS, beta-tubulin and the concatenated sequences were run with Kimura 2 parameters, using Gamma distribution model and treating gaps, and missing data as partial deletion. The ITS alignment consisted of 567, the beta-tubulin alignment of 376 and the concatenated alignment of 943 nucleotide positions. The bootstrap values were generated with 1000 replicates. Sequence similarity percentages was calculated with the Muscle method.
4.7. Secondary Metabolite Profiles
Fungal growth and metabolite screening was carried out according to Slack et al. [15] with some modifications. The isolates were inoculated on CY20S and plates were incubated at 25 °C for 7 days to obtain heavily sporulating cultures. Spore inoculums were prepared in sterile dH2O and used for inoculating Erlenmeyer flasks containing 100 mL of CY broth without 20% sucrose at a final concentration of 1 × 104 spores/mL. Static cultures were incubated in the dark at 25 °C for two weeks. After incubation, fungal cultures were filtered and a volume of 50 mL of crude filtrate was extracted twice with equal volumes of ethyl acetate in a 250 mL separatory funnel, concentrated under vacuum at 45 °C and 90 rpm, and dried under nitrogen gas. The residue was redissolved in 4 mL of methanol, filtered through a syringe filter (Acrodisc CR PTFE 0.45 µm, Gelman, USA) and analysed by HPLC-MS according to Nielsen and Smedsgaard [80]. The HPLC-MS system consisted of a Thermo Electron Corporation “Surveyor” equipped with a quaternary pump, an autosampler, ion trap (3D) (LCQ Advantage Max, Thermo Electron Corp., USA). The MS system was operated in the positive electrospray ionization (ESI) mode, N2 flow 10 L/min, voltage of 4.00 V positive, full scan over the mass range m/z 210–900 (single MS). The analytical column was a Thermo Scientific 50 × 2.1 mm 3 µm particle size. The injection volume was 10 µL. The analytical separation was performed using gradient elution with water (A) and acetonitrile (B) mobile phase, both modified with 0.1% formic acid. The following gradient was used: 85% A–15%B (initial), 0%–100% B (40 min), 100% B (5 min), 85% A–15% B (7 min), 85% A–15% B (2 min). The flow rate was 0.3 mL/min.
Acknowledgments
This work was supported by UNQ, UNRN, CONICET, and Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT).
Appendixes
Table A1.
ITS and beta-tubulin sequences used in the phylogenetic analyses.
| Species Name | Strain Number | GenBank Accession Number | Reference | |
|---|---|---|---|---|
| ITS | Beta Tubulin | |||
| A. montevidensis | NRRL 108 T | EF652077 | EF651898 | [64] |
| NRRL 35697 | EF652084 | EF651902 | [64] | |
| A. chevalieri | NRRL 78 T | EF652068 | EF651911 | [64] |
| NRRL 4755 | EF652071 | EF651913 | [64] | |
| A. cristatus | NRRL 4222 T | EF652078 | EF651914 | [64] |
| A. intermedius | NRRL 82 T | EF652074 | EF651892 | [64] |
| A. costiformis | CBS 101749 T | HE615136 | HE801320 | [42] |
| A. glaucus | NRRL 116 T | EF652052 | EF651887 | [64] |
| NRRL 120 | EF652054 | EF651889 | [64] | |
| A. niveoglaucus | NRRL 127 T | EF652058 | EF651905 | [64] |
| A. proliferans | NRRL 1908 T | EF652064 | EF651891 | [64] |
| NRRL 114 | EF652051 | EF651886 | [64] | |
| CCF 4146 | HE578067 | HE578076 | [42,81] | |
| IRAN 2088C TT | KC473922 | KC473925 | [48] | |
| IRAN 2089C | KC473923 | KC473926 | [48] | |
| A. brunneus | NRRL 131 T | EF652060 | EF651907 | [64] |
| A. neocarnoyi | NRRL 126 T | EF652057 | EF651903 | [64] |
| A. appendiculatus | CBS 374.75 T | HE615132 | HE801333 | [42] |
| A. cibarius | KACC 46346 T | JQ918177 | JQ918180 | [18] |
| A. pseudoglaucus | NRRL 40 T | EF652050 | EF651917 | [64] |
| NRRL 17 | EF652049 | EF651916 | [64] | |
| A. ruber | NRRL 52 T | EF652066 | EF651920 | [64] |
| NRRL 76 | EF652067 | EF651921 | [64] | |
| A. tonophilus | NRRL 5124 T | EF652081 | EF651919 | [64] |
| A. cumulatus | KACC 47316 T | KF928303 | KF928297 | [19] |
| A. xerophilus | NRRL 6131 T | EF652085 | EF651923 | [64] |
| A. leucocarpus | NRRL 3497 T | EF652087 | EF651925 | [64] |
| Penicillium steckii | NRRL 35625 | EF200085 | EF198551 | [82] |
T Ex-type strain, TT Ex-teleotype strain.
Table A2.
Extra information on the previous and actual nomenclature of the species and strains of Aspergillus section Aspergillus, used in the phylogenetic analyses.
| Actual Species Name | Strain Number | Previous Nomenclature |
|---|---|---|
| A. montevidensis | NRRL 108 T | E. amstelodami (Mangin) |
| NRRL 35697 | E. amstelodami (Mangin) | |
| A. chevalieri | NRRL 78 T | E. chevalieri (Mangin) |
| NRRL 4755 | E. chevalieri (Mangin) | |
| A. cristatus | NRRL 4222 T | E. cristatum (Raper & Fennell) Malloch & Cain |
| A. intermedius | NRRL 82 T | E. intermedium (Blaser) |
| A. costiformis | CBS 101749 T | A. costiformis |
| A. glaucus | NRRL 116 T | E. herbariorum (Link) |
| NRRL 120 | E. umbrosum | |
| A. niveoglaucus | NRRL 127 T | E. niveoglaucum |
| A. proliferans | NRRL 1908 T | A. proliferans |
| NRRL 114 | E. herbariorum * | |
| CCF 4146 | A. proliferans | |
| IRAN 2088C TT | A. proliferans | |
| IRAN 2089C | A. proliferans | |
| A. brunneus | NRRL 131 T | E. echinulatum |
| A. neocarnoyi | NRRL 126 T | E. carnoyi |
| A. appendiculatus | CBS 374.75 T | A. appendiculatus |
| A. cibarius | KACC 46346 T | A. cibarius |
| A. pseudoglaucus | NRRL 40 T | E. pseudoglaucum |
| NRRL 17 | E. repens | |
| A. ruber | NRRL 52 T | E. rubrum |
| NRRL 76 | E. rubrum | |
| A. tonophilus | NRRL 5124 T | E. tonophilum |
| A. cumulatus | KACC 47316 T | --- |
| A. xerophilus | NRRL 6131 T | E. xerophilum |
| A. leucocarpus | NRRL 3497 T | E. leucocarpum |
* Misidentified strain. According to Hubka et al. [42] study, the correct name of the strain is A. proliferans.T Ex-type strain, TT Ex-teleotype strain.
Author Contributions
G.P. and A.P. designed the study. M.G. and M.K. performed the experiments and analysed the data. All authors contributed to writing the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Hussein H.S., Brasel J.M. Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology. 2001;167:101–134. doi: 10.1016/S0300-483X(01)00471-1. [DOI] [PubMed] [Google Scholar]
- 2.Khosravi A.R., Dakhili M., Shokri H. A mycological survey on feed ingredients and mixed feeds in Ghom Province, Iran. Pak. J. Nutr. 2008;7:31–34. doi: 10.3923/pjn.2008.31.34. [DOI] [Google Scholar]
- 3.Shareef A.M. Molds and mycotoxins in poultry feeds farms of potential mycotoxicosis. Iraqi J. Vet. Sci. 2010;24:17–25. [Google Scholar]
- 4.Cegielska-Radziejewska R., Stuper K., Szablewski T. Micoflora and mycotoxin contamination in poultry feed mixtures from western Poland. Ann. Agric. Environ. Med. 2013;20:30–35. [PubMed] [Google Scholar]
- 5.Accensi F., Abarca M.L., Cabañes F.J. Occurrence of Aspergillus species in mixed feeds and component raw materials and their ability to produce ochratoxin A. Food Microbiol. 2004;21:623–627. doi: 10.1016/j.fm.2003.12.003. [DOI] [Google Scholar]
- 6.Samson R.A., Hoekstra E.S., Frisvad J.C., Filtenborg O. Introduction to Food- and Airborne Fungi. Centraalbureau voor Schimmelcultures; Utrecht, The Netherlands: 2000. [Google Scholar]
- 7.Butinar L., Zalar P., Frisvad J.C., Gunde-Cimerman N. The genus Eurotium—members of indigenous fungal community in hypersaline waters of salterns. FEMS Microbiol. Ecol. 2005;51:155–166. doi: 10.1016/j.femsec.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Pitt J.I., Hocking A.D. Fungi and Food Spoilage. 3rd ed. Springer; Berlin, Germany: 2009. [Google Scholar]
- 9.Al-Julaifi M.Z. Ochratoxin A production by Eurotium amstelodami and Eurotium spp. isolated from locally grown barley in Saudi Arabia. Kuwait J. Sci. Eng. 2003;30:59–66. [Google Scholar]
- 10.Wheeler K.A., Hocking A.D., Pitt J.I., Anggawati A.M. Fungi associated with Indonesian dried fish. Food Microbiol. 1986;3:351–357. doi: 10.1016/0740-0020(86)90020-1. [DOI] [Google Scholar]
- 11.Abellana M., Magrí X., Sanchis V., Ramos A.J. Water activity and temperature effects on growth of Eurotium amstelodami, E. chevalieri and E. herbariorum on a sponge cake analogue. Int. J. Food Microbiol. 1999;52:97–103. doi: 10.1016/S0168-1605(99)00131-2. [DOI] [PubMed] [Google Scholar]
- 12.Ohtsuki T., Yamada N., Kobori H., Osumi M. Identification of Eurotium (Aspergillus) restrictus group species by comparison of conidiospores on foxing using scanning electron microscope. J. Electron Microsc. 1992;41:270–272. [Google Scholar]
- 13.Florian M.L.E., Manning L. SEM analysis of irregular fungal fox spots in an 1854 book: Population dynamics and species identification. Int. Biodeter. Biodegr. 2000;46:205–220. doi: 10.1016/S0964-8305(00)00062-7. [DOI] [Google Scholar]
- 14.Ishii K., Hitosugi M., Kido M., Yaguchi T., Nishimura K., Hosoya T., Tokudome S. Analysis of fungi detected in human cadavers. Legal Med. 2006;8:188–190. doi: 10.1016/j.legalmed.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Slack G.J., Puniani E., Frisvad J.C., Samson R.A. Secondary metabolites from Eurotium species, Aspergillus calidoustus and A. insuetus common in Canadian homes with a review of their chemistry and biological activities. Mycol. Res. 2009;113:480–490. doi: 10.1016/j.mycres.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Kis-Papo T., Weig A.R., Riley R., Persoh D., Salamov A., Sun H., Lipzen A., Wasser S.P., Rambold G., Grigoriev I.V., Nevo E. Genomic adaptations of the halophilic Dead Sea filamentous fungus Eurotium Rubrum. Nat. Commun. 2013;5:1–8. doi: 10.1038/ncomms4745. [DOI] [PubMed] [Google Scholar]
- 17.Hong S.B., Kim D.H., Lee M., Baek S.Y., Kwon S.W., Samson R.A. Taxonomy of Eurotium species isolated from Meju. J. Microbiol. 2011;49:669–674. doi: 10.1007/s12275-011-0376-y. [DOI] [PubMed] [Google Scholar]
- 18.Hong S.B., Lee M., Kim D.H., Meijer M., Majoor E., van Kuyk P.A., Samson R.A. Aspergillus cibarius sp. nov., from traditional Meju in Korea. J. Microbiol. 2012;50:712–714. doi: 10.1007/s12275-012-2347-3. [DOI] [PubMed] [Google Scholar]
- 19.Kim D.H., Kim S.H., Kwon S.W., Lee J.K., Hong S.B. Aspergillus cumulatus sp. nov., from rice straw and air for meju fermentation. J. Microbiol. Biotechnol. 2014;24:334–336. doi: 10.4014/jmb.1312.12006. [DOI] [PubMed] [Google Scholar]
- 20.Ishikawa Y., Morimoto K., Hamasaki T. Metabolites of Eurotium species, their antioxidative properties and synergism with tocopherol. J. Food Sci. 1985;50:1742–1744. doi: 10.1111/j.1365-2621.1985.tb10579.x. [DOI] [Google Scholar]
- 21.Kanokmedhakul K., Kanokmedhakul S., Suwannatrai R., Soytong K., Prabpai S., Kongsaeree P. Bioactive meroterpenoids and alkaloids from the fungus Eurotium Chevalieri. Tetrahedron. 2011;67:5461–5468. doi: 10.1016/j.tet.2011.05.066. [DOI] [Google Scholar]
- 22.Gao J., Radwan M., León F., Wang X., Jaboc M., Tekwani B.L., Khan S.I., Lupien S., Hill R.A., Dugan F.M., et al. Antimicrobial and antiprotozoal activities of secondary metabolites from the fungus Eurotium repens. Med. Chem. Res. 2012;10:3080–3086. doi: 10.1007/s00044-011-9798-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Umeda M., Yamashita T., Saito M., Sekita S., Takahashi C. Chemical and cytotoxicity survey on the metabolites of toxic fungi. J. Exp. Med. 1974;44:83–96. [PubMed] [Google Scholar]
- 24.Bachmann M., Luethy J., Schlatter C. Toxicity and mutagenicity of molds of the Aspergillus glaucus group. Identification of physcion and three related anthraquinones as main toxic constituents from Aspergillus chevalieri. J. Agric. Food Chem. 1979;27:1342–1347. doi: 10.1021/jf60226a021. [DOI] [PubMed] [Google Scholar]
- 25.Nazar M., Ali M., Fatima T., Gubler C.J. Toxicity of flavoglaucin from Aspergillus chevalieri in rabbits. Toxicol. Lett. 1984;23:233–237. doi: 10.1016/0378-4274(84)90132-2. [DOI] [PubMed] [Google Scholar]
- 26.Ali M., Mohammed N., Alnaqeeb M.A., Hassan R.A., Ahmad H.S. Toxicity of echinulin from Aspergillus chevalieri in rabbits. Toxicol. Lett. 1989;48:235–241. doi: 10.1016/0378-4274(89)90049-0. [DOI] [PubMed] [Google Scholar]
- 27.Leitao J., de Saint-Blanquat G., Bailly J.R. Action of phosphine on production of aflatoxins by various Aspergillus strains isolated from foodstuffs. App. Environ. Microbiol. 1987;53:2328–2331. doi: 10.1128/aem.53.10.2328-2331.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jayaraman P., Kalyanasundaram I. Natural occurrence of toxigenic fungi and mycotoxins in rice bran. Mycopathologia. 1990;110:81–85. doi: 10.1007/BF00446995. [DOI] [PubMed] [Google Scholar]
- 29.El-Kady I., el-Maraghy S., Zohri A.N. Mycotoxin producing potential of some isolates of Aspergillus flavus and Eurotium groups from meat products. Microbiol. Res. 1994;149:297–307. doi: 10.1016/S0944-5013(11)80073-X. [DOI] [PubMed] [Google Scholar]
- 30.Abarca M.L., Bragulat M.R., Castellá G., Accensi F., Cabañes F.J. Hongos productores de micotoxinas emergentes. Rev. Iberoam. Micol. 2000;17:S63–S68. [PubMed] [Google Scholar]
- 31.Fraga M.E., Curvello F., Gatti M.J., Cavaglieri L.R., Dalcero A.M., da Rocha Rosa C.A. Potential aflatoxin and ochratoxin A production by Aspergillus species in poultry feed processing. Vet. Res. Commun. 2007;31:343–353. doi: 10.1007/s11259-006-3434-x. [DOI] [PubMed] [Google Scholar]
- 32.Frisvad J.C., Thrane U., Samson R.A., Hoekstra E.S. Mycotoxin production by common filamentous fungi. In: Samson R.A., Hoekstra E.S., Frisvad J.C., Filtenborg O., editors. Introduction to Food and Airborne Fungi. Centraalbureau voor Schimmelcultures; Utrecht, The Netherlands: 2002. pp. 321–330. [Google Scholar]
- 33.Frisvad J.C., Thrane U., Samson R.A. Mycotoxin producers. In: Dijksterhuis J., Samson R.A., editors. Food Mycology: A Multifaceted Approach to Fungi and Food. CRC Press; Boca Ratón, FL, USA: 2007. pp. 135–159. [Google Scholar]
- 34.Binder E.M., Tan L.M., Chin L.J., Handl J., Richard J. Worldwide occurrence of mycotoxins in commodities, feeds and feed ingredients. Anim. Feed Sci. Tech. 2007;137:265–282. doi: 10.1016/j.anifeedsci.2007.06.005. [DOI] [Google Scholar]
- 35.Venancio A., Paterson R. The challenge of mycotoxins. In: McElhatton A., Marshall R.J., editors. Food Safety—A Practical and Case Study Approach. Springer; Berlin, Germany: 2007. pp. 24–47. [Google Scholar]
- 36.Greco M.V., Pardo A.G., Ludemann V., Martino P.E., Pose G.N. Mycoflora and natural incidence of selected mycotoxins in rabbit and chinchilla feeds. Sci. World J. 2012;2012:1–6. doi: 10.1100/2012/956056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greco M.V., Franchi M.L., Rico Golba S.L., Pardo A.G., Pose G.N. Mycotoxins and Mycotoxigenic fungi in poultry feed for food-producing animals. Sci. World J. 2014;2014:1–9. doi: 10.1155/2014/968215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andersen B., Nielsen K.F., Fernández Pinto V., Patriarca A. Characterization of Alternaria strains from Argentinean blueberry, tomato, walnut and wheat. Int. J. Food Microbiol. 2015;196:1–10. doi: 10.1016/j.ijfoodmicro.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 39.McNeill J., Barrie F.R., Buck W.R., Demoulin V., Greuter W., Hawksworth D.L., Herendeen P.S., Knapp S., Marhold K., Prado J., et al. International Code of Nomenclature for algae, fungi, and plants (Melbourne Code) adopted by the Eighteenth International Botanical Congress Melbourne, Australia, July 2011. Regnum Veg. 2012;154:1–208. [Google Scholar]
- 40.Samson R.A., Visagie C.M., Houbraken J.J., Hong S.B., Hubka V., Klaassen C.H.W., Perrone G., Seifert K.A., Susca A., Tanney J.B., et al. Phylogeny, identification and nomenclature of the genus Aspergillus. Stud. Mycol. 2014;78:141–173. doi: 10.1016/j.simyco.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pitt J.I., Taylor J.W. Aspergillus, its sexual states and the new International Code of Nomenclature. Mycologia. 2014;106:1051–1062. doi: 10.3852/14-060. [DOI] [PubMed] [Google Scholar]
- 42.Hubka V., Kolarík M., Kubátová A., Peterson S.W. Taxonomic revision of Eurotium and transfer of species to Aspergillus. Mycologia. 2013;105:912–937. doi: 10.3852/12-151. [DOI] [PubMed] [Google Scholar]
- 43.Blaser P. Taxonomische und physiologische Untersuchungen über die Gatung Eurotium Link ex Fries. Sydowia. 1975;28:1–49. [Google Scholar]
- 44.Samson R.A. A compilation of the Aspergilli described since 1965. Stud. Mycol. 1979;18:1–38. [Google Scholar]
- 45.Domsch K.H., Gams W., Anderson T.H. Compendium of Soil Fungi. Volume 2 Academic Press; London, UK: 1980. [Google Scholar]
- 46.Tzean S.S., Chen J.L., Lious G.Y., Chen C.C., Hsu W.H. Aspergillus and related teleomorphs from Taiwan. CCRC Mycological Monographs FIRDI; Hsinchu, Taiwan: 1990. [Google Scholar]
- 47.Clarke J.H., Griffiths D.A. Ascospores of some common species of Eurotium (Aspergillus glaucus) as shown by scanning electron microscopy. Trans. Brit. Mycol. Soc. 1970;55:117–122. doi: 10.1016/S0007-1536(70)80102-4. [DOI] [Google Scholar]
- 48.Asgari B., Zare R., Zamanizadeh H.R., Rezaee S. Aspergillus osmophilus sp. nov., and a new teleomorph for A. proliferans. Mycoscience. 2014;55:53–62. doi: 10.1016/j.myc.2013.05.005. [DOI] [Google Scholar]
- 49.Visagie C.M., Hirooka Y., Tanney J.B., Whitfield E., Mwange K., Meijer M., Amend A.S., Seifert K.A., Samson R.A. Aspergillus, Penicillium and Talaromyces isolated from house dust samples collected around the world. Stud. Mycol. 2014;78:63–139. doi: 10.1016/j.simyco.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cardillo R., Fuganti C., Gatti G., Ghiringhelli D., Grasselli P. Molecular structure of cryptoechinuline A, a new metabolite of Aspergillus amstelodami, isolated during investigations on echinuline biosyntheis. Tetrahedron Lett. 1974;36:3163–3166. doi: 10.1016/S0040-4039(01)91850-7. [DOI] [Google Scholar]
- 51.Stipanovic R.D., Schroeder H.W. Preechinulin a metabolite of Aspergillus chevalieri. Trans. Brit. Mycol. Soc. 1976;66:178–179. doi: 10.1016/S0007-1536(76)80117-9. [DOI] [Google Scholar]
- 52.Cole R.J., Cox R.H. Tremorgen group. In: Cole R.J., Cox R.H., editors. Handbook of Toxic Fungal Metabolites. Academic Press, Inc.; New York, NY, USA: 1981. pp. 355–509. [Google Scholar]
- 53.Carll W.T., Forgacs J., Herring A.S. Toxicity of fungi isolated from a food concentrate. Am. J. Hyg. 1954;60:8–14. doi: 10.1093/oxfordjournals.aje.a119705. [DOI] [PubMed] [Google Scholar]
- 54.Schumaier G., Panda B., DeVolt H.M., Laffer N.C., Creek R.D. Hemorrhagic lesions in chickens resembling naturally occurring “hemorrhagic syndrome” produced experimentally by mycotoxins. Poultry Sci. 1961;40:1132–1134. doi: 10.3382/ps.0401132a. [DOI] [Google Scholar]
- 55.Rabie C.J., DeKlerk W.A., Terblanche M. Toxicity of Aspergillus amstelodami to poultry and rabbits. S. Afr. J. Agric. Sci. 1964;7:341–344. [Google Scholar]
- 56.Kinosita R., Shikato T. On toxic moldy rice. In: Wogan G.N., editor. Mycotoxins in Foodstuffs. MIT Press; Cambridge, MA, USA: 1965. pp. 111–132. [Google Scholar]
- 57.Semeniuk G., Harshfield G.S., Carlson C.W., Hesseltine C.W., Kwolek F. Mycotoxins in Aspergillus. Mycopathol. Mycol. Appl. 1971;43:137–152. doi: 10.1007/BF02051714. [DOI] [PubMed] [Google Scholar]
- 58.Wu M.T., Ayres J.C., Koehler P.E. Toxigenic Aspergilli and Penicillia isolated from aged cured meats. Appl. Microbiol. 1974;28:1094–1096. doi: 10.1128/am.28.6.1094-1096.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vesonder R.F., Lambert R., Wicklow D.T., Biehl M.L. Eurotium sp. and echinulin in feed refused by swine. Appl. Environ. Microbiol. 1988;54:830–831. doi: 10.1128/aem.54.3.830-831.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parshikov I.A., Miriyala B., Muraleedharan K.M., Avery M.A., Williamson J.S. Microbial transformation of artemisinin to 5-hydroxyartemisinin by Eurotium amstelodami and Aspergillus niger. J. Ind. Microbiol. Biot. 2006;33:349–352. doi: 10.1007/s10295-005-0071-2. [DOI] [PubMed] [Google Scholar]
- 61.Almeida A.P., Dethoup T., Singburaudom N., Lima R., Vasconcelos M.H., Pinto M., Kijjoa A. The in vitro anticancer activity of the crude extract of the sponge-associated fungus Eurotium cristatum and its secondary metabolites. J. Nat. Pharm. 2010;1:25–29. [Google Scholar]
- 62.Li D.L., Li X.M., Li T.G., Dang H.Y., Proksch P., Wang B.G. Benzaldehyde derivatives from Eurotium rubrum, an endophytic fungus derived from the mangrove plant Hibiscus tiliaceus. Chem. Pharm. Bull. 2008;56:1282–1285. doi: 10.1248/cpb.56.1282. [DOI] [PubMed] [Google Scholar]
- 63.Smetanina O.F., Kalinovskii A.I., Khudyakova Y.V., Slinkina N.N., Pivkin M., Kuznetsova T.A. Metabolites from the marine fungus Eurotium repens. Chem. Nat. Compd. 2007;43:395–398. doi: 10.1007/s10600-007-0147-5. [DOI] [Google Scholar]
- 64.Peterson S.W. Phylogenetic analysis of Aspergillus species using DNA sequences from four loci. Mycologia. 2008;100:205–226. doi: 10.3852/mycologia.100.2.205. [DOI] [PubMed] [Google Scholar]
- 65.Kozakiewicz Z. Aspergillus species on stored products. Myc. Papers. 1989;161:1–188. [Google Scholar]
- 66.Yun Y.H., Hyun M.W., Suh D.Y., Kim Y.M., Kim S.H. Identification and characterization of Eurotium rubrum isolated from Meju in Korea. Mycobiology. 2009;37:251–257. doi: 10.4489/MYCO.2009.37.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schoch C.L., Seifert K.A., Huhndorf S., Robert V., Spouge J.L., Levesque C.A., Chen W., Fungal Barcoding Consortium Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA. 2012;109:6241–6246. doi: 10.1073/pnas.1117018109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Geiser D.M., Klich M.A., Frisvad J.C., Peterson S.W., Varga J., Samson R.A. The current status of species recognition and identification in Aspergillus. Stud. Mycol. 2007;59:1–10. doi: 10.3114/sim.2007.59.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Visagie C.M., Varga J., Houbraken J., Meijer M., Kocsubé S., Yilmaz N., Fotedar R., Seifert K.A., Frisvad J.C., Samson R.A. Ochratoxin production and taxonomy of the yellow aspergilli (Aspergillus section Circumdati) Stud. Mycol. 2014;78:1–61. doi: 10.1016/j.simyco.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yazdani D., Zainal Abidin M.A., Tan Y.H., Kamaruzaman S. Molecular identification of Aspergillus and Eurotium species isolated from rice and their toxin-producing ability. Microbiology. 2011;80:720–727. doi: 10.1134/S0026261711050195. [DOI] [PubMed] [Google Scholar]
- 71.Speijersa G.J.A., Speijersb M.H.M. Combined toxic effects of mycotoxins. Toxicol. Lett. 2004;153:91–98. doi: 10.1016/j.toxlet.2004.04.046. [DOI] [PubMed] [Google Scholar]
- 72.Šegvić Klarić M. Adverse effects of combined mycotoxins. Arh. Hig. Rada Toksikol. 2012;63:519–530. doi: 10.2478/10004-1254-63-2012-2299. [DOI] [PubMed] [Google Scholar]
- 73.Wang H.W., Wang J.Q., Zheng B.Q., Li S.L., Zhang Y.D., Li F.D., Zheng N. Cytotoxicity induced by ochratoxin A, zearalenone, and α-zearalenol: effects of individual and combined treatment. Food Chem. Toxicol. 2014;71:217–224. doi: 10.1016/j.fct.2014.05.032. [DOI] [PubMed] [Google Scholar]
- 74.Hocking A.D., Pitt J.I. Dichloran-glycerol medium for enumeration of xerophilic fungi from low moisture foods. Appl. Environ. Microb. 1980;39:488–492. doi: 10.1128/aem.39.3.488-492.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.González H.H., Resnik S.L., Boca R.R., Marasas W.F. Mycoflora of Argentinian corn harvested in the main production area in 1990. Mycopathologia. 1995;130:29–36. doi: 10.1007/BF01104346. [DOI] [PubMed] [Google Scholar]
- 76.White T.J., Bruns T., Lee S., Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M.A., Gelfand D.H., Sninsky J.J., White T.J., editors. PCR Protocols: A Guide to Methods and Applications. Academic Press; New York, NY, USA: 1990. pp. 315–322. [Google Scholar]
- 77.Glass N.L., Donaldson G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microb. 1995;61:1323–1330. doi: 10.1128/aem.61.4.1323-1330.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA 6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Edgar R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nielsen K.F., Smedsgaard J. Fungal metabolite screening: database of 474 mycotoxins and fungal metabolites for dereplication by standardised liquid chromatography-UV-mass spectrometry methodology. J. Chromatogr. A. 2003;1002:111–136. doi: 10.1016/S0021-9673(03)00490-4. [DOI] [PubMed] [Google Scholar]
- 81.Hubka V., Kubatova A., Mallatova N., Sedlacek P., Melichar J., Skorepova M., Mencl K., Lyskova P., Sramkova B., Chudickova M., et al. Rare and new etiological agents revealed among 178 clinical Aspergillus strains obtained from Czech patients and characterized by molecular sequencing. Med. Mycol. 2012;50:601–610. doi: 10.3109/13693786.2012.667578. [DOI] [PubMed] [Google Scholar]
- 82.Serra R., Peterson S., CTCOR. Venâncio A. Multilocus sequence identification of Penicillium species in cork bark during plank preparation for the manufacture of stoppers. Res. Microbiol. 2008;159:178–186. doi: 10.1016/j.resmic.2007.12.009. [DOI] [PubMed] [Google Scholar]