Abstract
Lipid molecules are increasingly recognized as signals exchanged by organisms interacting in pathogenic and/or symbiotic ways. Some classes of lipids actively determine the fate of the interactions. Host cuticle/cell wall/membrane components such as sphingolipids and oxylipins may contribute to determining the fate of host–pathogen interactions. In the present field study, we considered the relationship between specific sphingolipids and oxylipins of different hybrids of Zea mays and fumonisin by F. verticillioides, sampling ears at different growth stages from early dough to fully ripe. The amount of total and free fumonisin differed significantly between hybrids and increased significantly with maize ripening. Oxylipins and phytoceramides changed significantly within the hybrids and decreased with kernel maturation, starting from physiological maturity. Although the correlation between fumonisin accumulation and plant lipid profile is certain, the data collected so far cannot define a cause-effect relationship but open up new perspectives. Therefore, the question—“Does fumonisin alter plant lipidome or does plant lipidome modulate fumonisin accumulation?”—is still open.
Keywords: maize, Fusarium verticillioides, sphingolipids, oxylipins, mycotoxin
1. Introduction
Maize is a globally-cultivated crop destined to food and feed production. However, maize is also highly prone to fungal contamination and specifically to mycotoxigenic fungi [1]. Among the fungal pathogens that infect maize, Fusarium verticillioides Sacc. (Nirenberg) is frequently the dominant toxigenic fungus found in temperate areas. In addition to causing seedling blight, root rot, stalk rot, kernel rot, or ear rot during maize colonisation, F. verticillioides produce fumonisins, toxic secondary metabolites causing a large variety of toxicological effects on animals and humans. Fumonisins are able to disrupt sphingoid based metabolism, and are suspected of inducing nervous system diseases in animals and humans [2,3]. Fumonisin B1 (FB1) has been included in the class 2B by the International Agency for Research on Cancer, because of its possible carcinogenic effect in humans [3]. In 2007, the European Commission defined thresholds for FB1 + FB2 content in raw maize and its derived products intended for human consumption (EU Commission Regulation No. 1126/2007). Recommendations have also been defined for animal feeding with differences between species (EU Commission Recommendation No. 576/2006) to prevent animal diseases like equine leukoencephalomalacia or acute pulmonary oedema in swine. Among the fumonisins synthesized by F. verticillioides, FB1, FB2 and recently FB3 are reported as the dominant ones in maize [4,5]; the presence of modified forms of B series fumonisins in raw maize, not detected by analytical methods commonly applied, was also reported [6,7].
Several studies explored the factors affecting mycotoxin synthesis and notably fumonisins. Recent studies have shown that the chemical composition of maize kernels can influence fumonisin content [8,9,10]; i.e., the composition of total fatty acids related closely to fumonisin accumulation in maize under field conditions. Notably, maize hybrids with high linoleic content displayed higher fumonisin contamination [8]. In general, lipid molecules are gaining momentum as crucial signals able to determine the fate of the interaction between cereals and mycotoxin-producing fungi [8,9,10,11]. In the plant cell, a particular class of lipid molecules, the sphingolipids (SL), are present in the cell membranes of all eukaryotes and play an important role during the interactions with the fungal pathogens [12]; ceramide (Cer), sphingosine (Sph), and sphingosine-1-phosphate (S1P) are part of this complex family of compounds. SL mediate different processes and a sharp modulation between Cer and S1P was highlighted. Notably, as the amount of Sph increases, the cell can initiate apoptosis or programmed cell death [13,14], whereas increases in S1P promote cell survival and proliferation [15]. In plants, SL concur to form the first line of defense against abiotic and biotic stresses. In particular, SL pre-cursors, i.e., long chain bases (LCB), increase upon pathogen infection [16]. Further, they are potentially cytotoxic for pathogenic microorganisms. As reported [17], fumonisins are potent inhibitors of ceramide synthases, the enzyme involved in sphingolipids synthesis either de novo or through salvage pathway. Persistent fumonisin inhibition of Cer biosynthesis resulted in the production of toxic free sphingoid bases, increases in sphingoid base 1-phosphates, depletion of critical membrane glycosphingolipids, and altered phospholipid signaling pathways in animals and in maize cells and seedlings [18,19].
Dall’Asta et al. 2015 [9] demonstrated by an untargeted lipidomic approach that four lipid entities might differentiate Zea mays hybrids highly contaminated with fumonisins (HFb) by the low-contaminated ones (LFb). In this paper, we have tested the reliability of these markers by planning an open field experiment on a subset of HFb hybrids. Notably, we considered the relationship between specific sphingolipids and fumonisin by F. verticillioides, sampling ears of different hybrids of Zea mays from early dough to fully ripe in open field.
2. Results
2.1. Incidence of Fungal Species, F. verticillioides and Fumonisin Production
Fungi were detected in all growth stages of kernel sampling, with an increasing trend towards harvest time. Contamination by Aspergillus spp. was found to be very limited (3% as mean of all samples) and Penicillium spp. was detected only sporadically, mainly at harvest (Table 1).
Table 1.
Results of the analysis of variance (ANOVA) and Tuckey test. The factors considered were: hybrid and growth stage (GS) (see Methods Section) and the variables were the incidence of kernels infected by fungi (Fungi), Aspergillus spp. (AI) and Fusarium spp. (FI), Free (FFB) and total (HFB) fumonisins B (B1 + B2 + B3) and oxylipins and ceramides detected in maize samples: 9-HODE, 13-HODE, N-(2ʹ-hydroxylignoceroyl)-phytosphingosine (HLP), N-lignoceroyl-phytosphingosine (LP).
| Factors | Fungi | AI | FI | FFB | TFB | 9-HODE | 13-HODE | HLP | PHY-CER | NOH |
|---|---|---|---|---|---|---|---|---|---|---|
| Hybrid | n.s. | n.s. | n.s. | ** | * | ** | * | ** | ** | ** |
| H17 | 47.0 | 4.7 | 24.9 | 4109.2 b | 4682.8 b | 7.4 ab | 10.9 | 2.3 ab | 2.7 ab | 2.3 b |
| H18 | 43.1 | 2.7 | 12.9 | 930.3 a | 531.8 a | 10.9 b | 11.3 | 2.9 b | 3.1 b | 2.8 b |
| H19 | 40.6 | 1.6 | 16.3 | 588.8 a | 411.1 a | 6.4 a | 8.6 | 1.9 a | 1.7 a | 1.3 a |
| H20 | 55.6 | 3.4 | 24.1 | 1943.2 ab | 2709.3 ab | 8.3 ab | 9.0 | 2.8 b | 1.8 a | 1.6 a |
| Sampling Time | ** | n.s. | ** | ** | ** | n.s. | ** | ** | ** | ** |
| 1 | 16.2 a | 7.9 | 7.4 a | 0.0 a | 0.0 a | 7.4 | 12.7 b | 2.8 b | 2.8 b | 2.9 b |
| 2 | 33.9 ab | 0.6 | 12.8 a | 481.4 a | 571.8 b | 9.7 | 9.8 ab | 2.8 b | 3.0 b | 2.4 b |
| 3 | 50.2 b | 3.9 | 16.4 a | 1690.6 b | 1734.7 b | 9.5 | 9.5 a | 2.2 ab | 2.4 b | 1.6 a |
| 4 | 85.9 c | 0.0 | 41.6 b | 5399.4 c | 6028.6 b | 6.4 | 7.8 a | 1.9 a | 1.1 a | 1.1 a |
n.s.: not significant; * p ≤ 0.05; ** p ≤ 0.01. a,b,c,ab different letters indicate significant differences among values.
Nevertheless, Aspergillus infection was limited. In some samples where it was present, no Fusarium strain was isolated (data not shown).
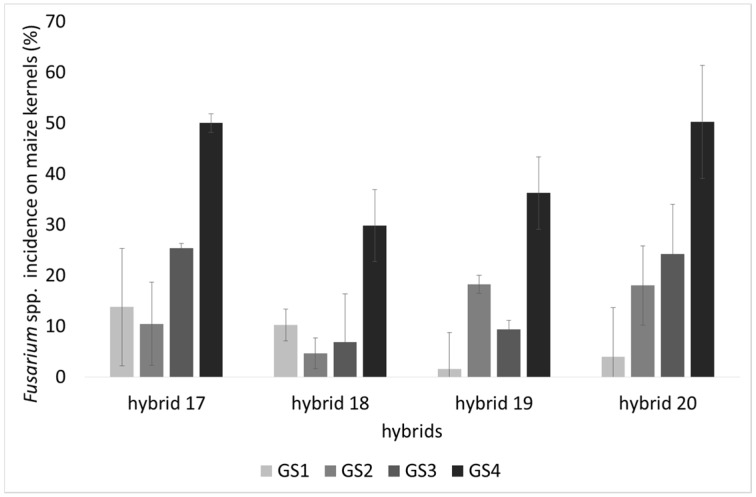
The incidence of kernels infected by Fusarium spp. significantly increased during the four growth stages (p < 0.001; Table 1). Notably, at GS4, hybrid 17 was found to be by far the most contaminated and hybrid 18 the least contaminated by Fusarium spp. (Mann Whitney Test; p < 0.01) (Figure 1).
Figure 1.
Fusarium spp. incidence (%) in naturally contaminated maize kernels from four commercial hybrids (17–20) harvested at four different growth stages: at early dough maturity (GS1), dough stage (GS2), physiological maturity (GS3) and fully ripe (GS4) in three locations in Italy. Results represent the mean of n = 9 incidence values deriving from the three different locations (biological replicates) in three technical replicates ± SE.
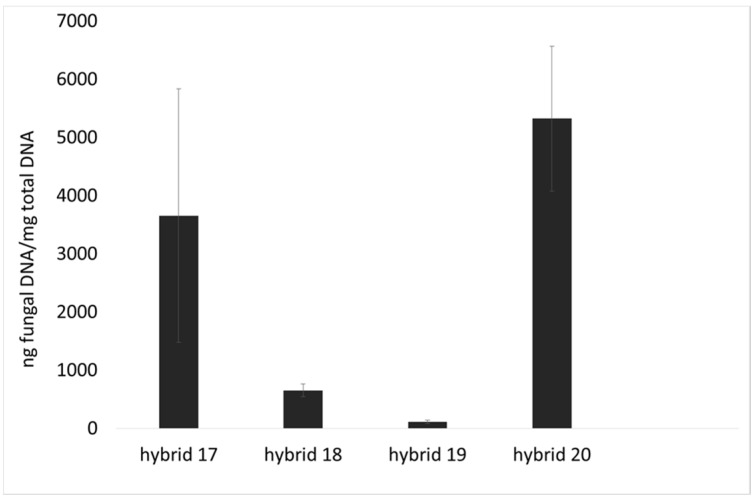
The amount of F. verticillioides DNA was measured at the fully ripe stage (GS4) by qPCR assay. A lower amount of F. verticillioides DNA was found in hybrids 18 and 19 compared to hybrid 17 (p < 0.05) and hybrid 20 (p < 0.001; Figure 2), in agreement with incidence data (Figure 1).
Figure 2.
Abundance of fungal DNA (ng fungal DNA/μg total DNA) as a measure of F. verticillioides colonization of maize ears at fully ripe stage (GS4) from four commercial hybrids (17–20) cultivated in three locations in Italy. Results represent the mean of n = 6 DNA amount values deriving from the three different locations (biological replicates) in two technical replicates ± SE.
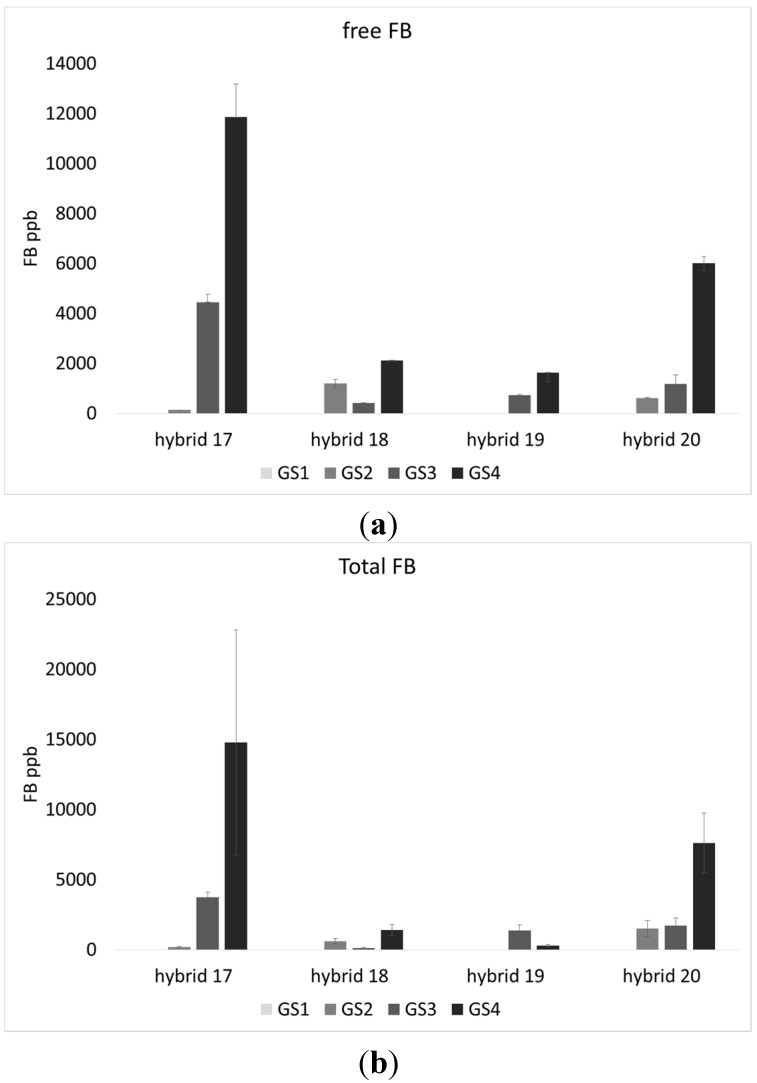
The total amount of fumonisin, in free and modified forms, differed significantly between hybrids and increased significantly with maize ripening. The lowest contamination was detected in hybrid 18 and 19, while hybrid 17 was the most contaminated both considering free and total fumonisins. It can be pinpointed that, as well as for fungal incidence, fumonisins, (a) free; and (b) total forms, increased in almost all the hybrids during the growth stages (Table 1; Figure 3a,b). The interaction between the hybrid and growth stage was not found to be significant for both fungi and fumonisin (data not shown).
Figure 3.
(a) Free; and (b) Hidden fumonisins (FB = FB1 + 2 + 3) content (ppb) in four maize hybrids, cultivated in three different localities and harvested at four different growth stages (GS1–4). Results represent the mean of n = 9 FB amount values deriving from the three different locations (biological replicates) in three technical replicates ± SE.
2.2. Lipid Profile of Maize Challenged with F. verticillioides at the Field Level
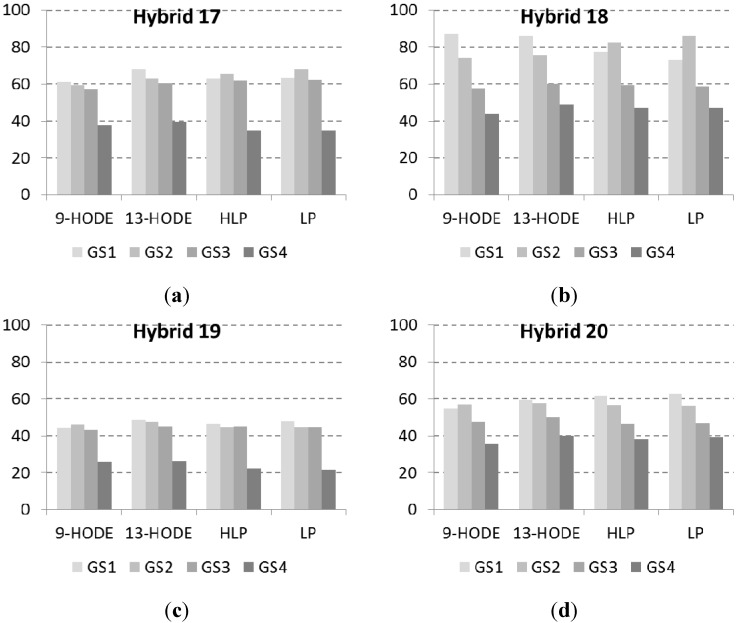
In relation to findings of [9], we focused our attention on the presence of specific fatty acid derivatives such as 9- and 13-HydroxyOctaDecEnoic acids (HODE), and phytoceramides such as lignoceric phytosphingosine (Cer(t18:0/24:0)) (LP) and hydroxyl-lignoceric phytosphingosine (Cer(t18:0/24:0(2OH))) (HLP) in the four commercial hybrids of maize naturally infected by F. verticillioides. 9-HODE and phytoceramides were significantly affected by hybrids, with hybrid 19 having always the lowest and hybrid 18 always the highest content. Fatty acid derivatives and phytoceramides decreased during kernel maturation. Specifically, the decrease started from the physiological maturity stage in hybrid 18 and hybrid 20, whilst it started from the fully ripe stage in hybrid 17 and hybrid 19 (Figure 4a–d), whereas 9-HODE was found to be unaffected by the growth stage (Table 2).
Figure 4.
(a–d) N-(2ʹ-hydroxylignoceroyl)-phytosphingosine (HLP), N-lignoceroyl-phytosphingosine (LP), 9- and 13-HODE, expressed as percentage on the maximum amount detected in each growing location, in hybrid 17 (a); hybrid 18 (b); hybrid 19 (c) and hybrid 20 (d) harvested at different growth stages: early dough maturity (GS1), dough stage (GS2), physiological maturity (GS3) and fully ripe (GS4). Results represent the mean of n = 9 values deriving from the three different locations (biological replicates) in three technical replicates ± SE.
Table 2.
Pearson correlation coefficients amongst the variables in four hybrids of maize cultivated in three different Italian locations calculated on 48 observations. The variables considered are growth stage (GS) (see Methods Section), oxylipins and ceramides detected in maize samples: 9-HODE, 13-HODE, N-(2ʹ-hydroxylignoceroyl)-phytosphingosine (HLP), N-lignoceroyl-phytosphingosine (LP), free (FFB) and Total (TFB) fumonisins B (B1 + B2 + B3) and the percentage of Fusarium infection on maize ears (FI).
| Variable | 13-HODE | HLP | LP | FFB | TFB | FI |
|---|---|---|---|---|---|---|
| 9-HODE | 0.371 c | 0.482 c | 0.205 a | −0.095 | −0.082 | −0.130 |
| 13-HODE | 0.683 c | 0.715 c | −0.208 a | −0.107 | −0.381 c | |
| HLP | 0.805 c | −0.221 a | −0.185 | −0.413 c | ||
| LP | −0.226 a | −0.179 | −0.416 c | |||
| TFB | 0.976 c | 0.491 c | ||||
| HFB | 0.309 b |
a: p < 0.05; b: p < 0.01; c: p < 0.001.
2.3. Correlations
The Pearson correlation was applied as a measure of the correlation between variables: oxylipins and ceramides detected in maize samples: 9-HODE, 13-HODE, HLP, LP, free (FFB) and total (TFB) fumonisins and the percentage of Fusarium infection in maize ears (FI). Pearson coefficients calculated from the whole dataset (“All Hybrids” = 48 observations) are shown in Table 2. The production of fumonisins (free forms), as well as fungal contamination, are negatively correlated with 13-HODE, HLP, LP biosynthesis.
3. Discussion
Metabolite profiling under field conditions is becoming increasingly popular. Several biotic and abiotic stresses affect plant metabolomes, which is rapidly becoming a central topic in metabolite-profiling experiments in the field [20]. The environment may buffer different stresses [21,22] and, in relation to this, the alteration of the plant metabolic profile under field conditions is more suggestive of its modification occurring under ex vivo conditions, e.g., in phytochambers [23]. Dall’Asta et al. [9] indicated, by a lipidomic approach applied in an open field study, that some phytoceramides distinguish maize hybrids conducive for fumonisin synthesis from non-conducive ones. Thus, in the present study, we planned open field experiments in three areas of Northern Italy, for evaluating the trend of these lipid markers in four different hybrids and the correlation with fumonisin accumulation.
As expected, almost all the lipid markers varied significantly between hybrids. In particular, in hybrid 19, the lowest amount was always detected, and, as such, so was the lowest fumonisin contamination. Besides, FFB were significantly and negatively correlated to 13-HODE, HLP and LP. This confirms the correlation of lipid derivatives’ content, including phytoceramides, in maize hybrids and fumonisin production. The few field studies, therefore, highlighted the role of plants in the regulation of fumonisin production under natural field conditions.
Fumonisins are inhibitor of ceramide synthases, enzymes which, starting from sphingosine, sphinganine and fatty acids, produce ceramides and, in plant, phytoceramides [24]. The inhibition of ceramide synthase alter the sphingolipid metabolism that, in turn, could trigger apoptosis, as demonstrated in mammalian [25].
Novel insights, deriving from genetic and biochemical studies, indicate that free sphingosines, as well as ceramides and their respective phosphorylated forms, play a critical role in the regulation of plant defence [15].
In mammals, bacterial and viral pathogens target predominantly structural and signalling lipids to alter the cellular phenotype of the host cell. On the contrary, fungal pathogens have complex lipidomes themselves and target predominantly the release of polyunsaturated fatty acids from the host cell lipidome. Authors reported that “eukaryotic pathogens focus on interference with lipid metabolites affecting systemic inflammatory reactions that are part of the host immune system” [26].
Desjardins et al. [27] correlated fumonisin-induced diseases in animals with the biochemical consequences of ceramide synthase inhibition. Over recent years, Proctor’s group indicated that fumonisin production by F. verticillioides is required for developing full symptoms of foliar disease in maize seedlings [19]. Moreover, these authors suggested, “the overall incidence and severity of seedling disease development are likely dependent on both maize genotype and the amount of fumonisin produced by F. verticillioides strains”. According to Williams et al. [2], fumonisins cause an important cascade of events leading to altered cell growth, differentiation, and cell injury. These events occurred in vitro as well as in vivo. Therefore, these authors propose that a prolonged exposure of maize plant to fumonisins resulted in a change of expression or relative activity of the enzymes responsible for metabolizing free sphingoid bases. Sanchez-Rangel et al. [28] further confirm the involvement of fumonisin production in fungal virulence showing that F. verticillioides and exogenously added FB1 down-modulate maize β-1,3-glucanases (PR2-like proteins). In Arabidopsis, FB1 might trigger plant defense hallmarks such as ROS production, phenolics and callose deposition, biosynthesis of phytoalexins, plus the expression of PR proteins and the activation of the jasmonate/ethylene and SA-dependent signaling pathways also related to PCD (Programmed Cell Death) onset [15]. Berkey et al. [15] propose that the sphinganine-like toxins, such as FB1, may induce PCD in plant by inhibiting acyl-CoA-dependent ceramide synthases. The production of fumonisins may result as part of a strategy exploited during the necrotrophic phase of F. verticillioides and suggests a close relation between sphingolipid metabolism and plant PCD. Regarding this, F. verticillioides could foster mycotoxin accumulation in maize, profoundly altering the sphingolipid metabolism.
Starting from these evidences, we used field studies for verifying at real scale the correlation between the fumonisins’ biosynthesis by F. verticilllioides and the synthesis of some phytoceramides during its interaction with the host. Our data show a negative correlation between lignoceric acid sphingosine and its hydroxylated forms, and the amount of fumonisin and fungal infection. These results are confirmed in three different growing locations, which provides consistency to the output.
The observed decrease in these ceramides could cause an increase in their related phytosphingosine moiety (t18:0). Several studies report that a rapid accumulation of endogenous dihydrosphingosine (d18:0) and t18:0 occurs within hours after FB1 treatment. This increase in d/t18:0 was correlated with PCD onset in plants as well as in animals [15].
In our study, we may hypothesise that in the hybrids conducive to fumonisins, the ceramide synthases are affected and a decrease in the amount of two specific phytoceramides occurs. This event could lead to an increase in t18:0 which, in turn, favours fungal growth inducing PCD, as stated by other authors [15]. We also confirm a close relationship between the whole lipid profile of maize kernels and fumonisin accumulation [8,9]. In relation to this, we also proved in this study that 13-HODE, a LOX-oxylipin, decreases significantly during the growing season, i.e. with the increase of fungal infection and fumonisin production. Thus, the ability of this pathogen to grow on maize ears is probably related with the reduction of this defense oxylipin.
Although fumonisin accumulation and the plant lipid profile are correlated, the data collected so far cannot define a cause–effect relationship. Therefore, the question—“Does fumonisin alter the plant lipidome or does the plant lipidome modulate fumonisin accumulation?”—is still open.
In conclusion, our data shows that, as suggested by Glenn et al., [19], both maize genotype and fumonisin play a role in the disease, but the role of genotype seems to be more important under field conditions.
4. Materials and Methods
4.1. Maize Sample Collection
Samples from four commercial hybrids were collected during the growing season in diverse locations of Northern Italy: Cremona, Pordenone and Torino. Sampling was carried out every two weeks, from maize early dough stage up to harvest time, therefore four times per field (growth stages 1–4; GS) indicatively, following the BBCH-identification keys of maize, corresponding to BBCH83 early dough (GS1), BBCH85 dough (GS2), BBCH87 physiological maturity (GS3), BBCH 89 fully ripe (GS4) [29,30].
Hybrids belong to different FAO classes: FAO 500 (one hybrid) and FAO 600 (three hybrids). The hybrid names were not reported because only a limited number of hybrids were considered and because the aim was to find out relevant factors not related to a specific hybrid, but of more general value, having in mind the short commercial life of hybrids. The used hybrids, commercially available in Italy, were reported with increasing numbers from hybrid 17 to 20 according to sample codification used in previous studies [8,9]. Ten ears were collected from three different rows chosen longitudinally along the field area; 30 ears were sampled for each hybrid. After husk elimination, ears were shelled and kernels of each sample were shared in two sub-samples of about 1 kg used for mycological and chemical-molecular analysis, respectively. Samples destined to chemical and molecular analysis were stored at −20 °C while the others were immediately processed.
4.2. Incidence of Infected Kernels
Fifty kernels were randomly selected from each sample and surface disinfected in 1% sodium hypochlorite solution for 2 min and then in 90% ethyl alcohol solution for 2 min. Kernels were rinsed with sterile distilled water and dried under a sterile hood. Grain kernels were plated on Petri dishes (Ø 9 cm) containing Potato Dextrose Agar (PDA, Oxoid Ltd., Basingstoke, Hampshire, UK) added with 0.1% streptomycin (Sigma-Aldrich, St. Louis, MO, USA) and incubated at 25 °C for 7 days with a 12 h light photoperiod. After incubation, kernels showing mould development were counted. The identification of growing colonies at genus level was done for Fusarium, Aspergillus and Penicillium [31,32,33]. Data obtained were used to calculate the incidence of fungi and of the above-mentioned genera on plated kernels.
4.3. Fungal Growth by qPCR
In order to monitor fungal growth in maize cobs, a specific SYBR green qPCR method was set by using FUM1 primers [10]. Real-time PCR was prepared in a 20 μL reaction mixture as described in [10].
4.4. Chemicals
Authentic oxylipins 9-hydroxy-10E,12Z-octadecadienoic acid (9-HODEd4, MW 300.5) and 13-hydroxy-9Z,11E-octadecadienoic acid (13-HODEd4, MW 300.5) were purchased from Cayman Chemicals (Ann Arbor, MI, USA) whereas authentic sphingolipids, N-(2ʹ-hydroxylignoceroyl)-phytosphingosine (Cer (t18:0/24:0(2OH), MW 683.6)) and N-lignoceroyl-phytosphingosine (Cer (t18:0/24:0), MW 667.6)) and N-palmitoyl-d31-d-erythro-sphingosine (C16-D31 Ceramide, MW 568.7) were purchased from Avanti polar lipids, Inc. (Alabaster, AL, USA).
4.5. Lipid Analysis
Lipid extraction and chromatography conditions were selected according to [10]. Deuterated compounds, such as 9-HODE d4, 13-HODE d4, and C16-d31 ceramide were used as the internal standards (ISTD). The amount of each ISTD added to the matrix before the extraction was 400 pmol, which corresponded to the final concentration of 2 µM when the dried extracts were dissolved in the final volume of 200 µL of MeOH. The analysis of lipid extracts was performed according to the previously reported method [10]. Briefly, lipid extracts were analyzed by rapid resolution reversed phase HPLC (RR-RP-HPLC) coupled with a triple quadrupole (QqQ) mass spectrometer (G6410A series triple quadrupole, QqQ, Agilent Technologies, Santa Clara, CA, USA) following ESI ionization as reported in [9] with slight modifications. In particular, negative and positive detection modes were selected for oxylipins and sphingolipids, respectively. In the first time segment, 0–3 min oxylipins were acquired in ESI negative ion mode, 3–38 min phytoceramides were acquired in ESI positive ion mode. Switch of polarity to positive ion mode to detect sphingolipids was set at 3 min. To acquire oxylipins and sphingolipids dynamic, multiple reaction monitoring (dMRM) experiments were performed [34]. The MRM transitions (Table 3), acquired by injecting authentic standards, were in agreement with the literature [33]. Relative abundance of each oxylipin (9 and 13-HODE), phytoceramide (N-(2ʹ-hydroxylignoceroyl)-phytosphingosine (HLP) and N-lignoceroyl-phytosphingosine (LP)) was expressed as the ratio between the compound and the ISTD transition. MRM data were processed using Mass Hunter Quantitative software (B.03.02 version, Agilent Technologies, Santa Clara, CA, USA).
Table 3.
Mass transitions, collision energy (CE), fragmentor energy and retention times of the lipid entities analyzed.
| Species | Precursor/Product Ion m/z | CE (eV) | Fragmentor (eV) | RT (min) |
|---|---|---|---|---|
| 9-HODE d4 (ISTD) | 299.2–>172.2 | −20 | −140 | 1.22 |
| 9-HODE | 295.2–>171.2 | −20 | −140 | 1.22 |
| 13-HODE d4 (ISTD) | 299.2–>198.2 | −20 | −140 | 1.23 |
| 13-HODE | 295.2–>195.2 | −20 | −140 | 1.23 |
| C16-D31 Ceramide (ISTD) | 568.6–>264.2 | +30 | +140 | 15.8 |
| Cer (t18:0/24:0(2OH)) | 684.6–>282.2 | +34 | +140 | 17.95 |
| Cer (t18:0/24:0) | 668.6–>282.2 | +34 | +140 | 18.35 |
4.6. Free and Total Fumonisins
Free and total fumonisins were determined according to our previous works [6,7,8,9]; each of these papers add an additional step to the full method we used for this work. Fumonisins obtained after sample hydrolysis were measured as the sum of hydrolyzed FB1, FB2, and FB3. All of the results are expressed as the sum of FB1, FB2, and FB3 equivalents, considering a correction factor due to the different molecular weights of parent and hydrolyzed compounds, and referred to as “total fumonisins” (FBtot).
4.7. Statistics
The analysis of variance (ANOVA) was applied to define the role of hybrids and their growth stage on all the parameters considered using growing locations (n = 3) as replicates; means were compared using the Tukey test. Fumonisin and lipid compounds data were rated according to the maximum amount detected per growing area and arcsen transformed before statistical analysis.
Two-paired comparisons were performed by Mann-Whitney Test and relative significance (p) calculated accordingly. The relationships between some variables were also tested for linearity calculating the relevant Pearson (p) correlation coefficients.
Data analysis was managed with PASW statistics 21 (ver. 21, SPSS Inc., Chicago, IL, USA, 2012).
Acknowledgments
The Ministry of Research and Education funded the present study through the project FIRB2008 “Futuro in Ricerca”, grant N. FIRB-RBFR08JKHI.
Abbreviations
| Cer | Ceramide |
| Free FB | Free fumonisins B |
| Hidden FB | Hidden fumonisins B |
| FB1 | fumonisin B1 |
| HODE | hydroxyoctadecenoic acids |
| MRM | multiple reaction monitoring |
| HLP | N-(2ʹ-hydroxylignoceroyl)-phytosphingosine |
| LP | N-lignoceroyl-phytosphingosine |
| qPCR | quantitative PCR |
| ROS | reactive oxygen species |
| Sph | sphingosine |
| S1P | sphingosine-1-phosphate |
| FBtot | total fumonisins |
| QqQ | triple quadrupole mass spectrometer |
Author Contributions
P. Giorni conceived and designed the experiments, performed the experiments and critical revision of the paper; C. Dall’Asta performed the experiments and critical revision of the paper; M. Reverberi conceived and designed the experiments, wrote the paper, critical revision of the paper; V. Scala performed the experiments, wrote the paper, critical revision of the paper; M. Ludovici conceived and designed the experiments, performed the experiments; M. Cirlini performed the experiments; G. Galaverna critical revision of the paper; C. Fanelli critical revision of the paper; P. Battilani analyzed the data, conceived and designed the experiments, critical revision of the paper.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- 1.Woloshuk C.P., Shim W.B. Aflatoxins, fumonisins, and trichothecenes: A convergence of knowledge. FEMS Microbiol. Rev. 2013;37:94–109. doi: 10.1111/1574-6976.12009. [DOI] [PubMed] [Google Scholar]
- 2.Williams L.D., Glenn A.E., Zimeri A.M., Bacon C.W., Smith M.A., Riley R.T. Fumonisin disruption of ceramide biosynthesis in maize roots and the effects on plant development and Fusarium verticillioides-induced seedling disease. J. Agric. Food Chem. 2007;55:2937–2946. doi: 10.1021/jf0635614. [DOI] [PubMed] [Google Scholar]
- 3.IARC . Monographs on the Evaluation of Carcinogenic Risks to Humans, Beryllium, Cadmium, Mercury, and Exposures in the Glass Manufacturing Industry. Volume 58. IARC; Lyon, France: 1993. [PMC free article] [PubMed] [Google Scholar]
- 4.Lazzaro I., Falavigna C., Dall’Asta C., Galaverna G., Battilani P. Fumonisins B, A and C profile and masking in Fusarium verticillioides strains on fumonisin-inducing and maize-based media. Int. J. Food Microbiol. 2012;159:93–100. doi: 10.1016/j.ijfoodmicro.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 5.Lazzaro I., Falavigna C., Galaverna G., Dall’Asta C., Battilani P. Cornmeal and starch influence the dynamic of fumonisin B, A and C production and masking in Fusarium verticillioides and F. proliferatum. Int. J. Food Microbiol. 2013;166:21–27. doi: 10.1016/j.ijfoodmicro.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Dall’Asta C., Mangia M., Berthiller F., Molinelli A., Sulyok M., Schumacher R., Krska R., Galaverna G., Dossena A., Marchelli R. Difficulties in fumonisin determination: The issue of hidden fumonisins. Anal. Bioanal. Chem. 2009;395:1335–1345. doi: 10.1007/s00216-009-2933-3. [DOI] [PubMed] [Google Scholar]
- 7.Dall’Asta C., Falavigna C., Galaverna G., Dossena A., Marchelli R. In vitro digestion assay for determination of hidden fumonisins in maize. J. Agric. Food Chem. 2010;58:12042–12047. doi: 10.1021/jf103799q. [DOI] [PubMed] [Google Scholar]
- 8.Dall’Asta C., Falavigna C., Galaverna G., Battilani P. Role of maize hybrids and their chemical composition in Fusarium infection, fumonisin production and masking. J. Agric. Food Chem. 2012;60:3800–3808. doi: 10.1021/jf300250z. [DOI] [PubMed] [Google Scholar]
- 9.Dall’Asta C., Giorni P., Cirlini M., Reverberi M., Gregori R., Ludovici M., Camera E., Fanelli C., Battilani P., Scala V. Maize lipids play a pivotal role in the fumonisin accumulation. World Mycotoxin J. 2014;8:87–97. doi: 10.3920/WMJ2014.1754. [DOI] [Google Scholar]
- 10.Scala V., Giorni P., Cirlini M., Ludovici M., Visentin I., Cardinale F., Fabbri A.A., Fanelli C., Reverberi M., Battilani P., et al. LDS1-produced oxylipins are negative regulators of growth, conidiation and fumonisin synthesis in the fungal maize pathogen Fusarium verticillioides. Front. Microbiol. 2014;5 doi: 10.3389/fmicb.2014.00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christensen A.S., Nemchenko A., Park Y.S., Borrego E., Huang P.C., Schmelz E.A., Kunze S., Feussner I., Yalpani N., Meeley R., et al. The novel monocot-specific 9-lipoxygenase ZmLOX12 is required to mount an effective jasmonate-mediated defense against Fusarium verticillioides in maize. Mol. Plant Microbe Interact. 2014;27:1263–1276. doi: 10.1094/MPMI-06-13-0184-R. [DOI] [PubMed] [Google Scholar]
- 12.Konig S., Feussner K., Schwarz M., Kaever A., Iven T., Landesfeind M., Ternes P., Karlovsky P., Lipka V., Feussner I. Arabidopsis mutants of sphingolipid fatty acid a-hydroxylases accumulate ceramides and salicylates. New Phytol. 2012;196:1086–1097. doi: 10.1111/j.1469-8137.2012.04351.x. [DOI] [PubMed] [Google Scholar]
- 13.Hannun Y.A., Obeid L.M. Principles of bioactive lipid signalling: Lessons from sphin-golipids. Nat. Rev. Mol. Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 14.Van Brocklyn J.R., Williams J.B. The control of the balance between ceramide and sphingosine-1-phosphate by sphingosine kinase: Oxidative stress and the seesaw of cell survival and death. Comp. Biochem. Physiol. 2012;163:26–36. doi: 10.1016/j.cbpb.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Berkey R., Bendigeri D., Xiao S. Sphingolipids and plant defense/disease: The “death” connection and beyond. Front. Plant Sci. 2012;3 doi: 10.3389/fpls.2012.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guillas I., Puyaubert J., Baudouin E. Nitric oxide sphingolipid interplays in plant signaling: An enigma from the Sphinx? Front. Plant Sci. 2013;4 doi: 10.3389/fpls.2013.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pewzner-Jung Y., Ben-Dor S., Futerman A.H. When do Lasses (longevity assurance genes) become CerS (ceramide synthases)? Insights into the regulation of ceramide synthesis. J. Biol. Chem. 2006;281:25001–25005. doi: 10.1074/jbc.R600010200. [DOI] [PubMed] [Google Scholar]
- 18.Zitomer N.C., Mitchell T., Voss K.A., Bondy G.S., Pruett S.T., Garnier-Amblard E.C., Liebeskind L.S., Park H.E., Wang M.C., Sullards A H., Jr., et al. Ceramide Synthase Inhibition by Fumonisin B1 Causes Accumulation of 1-Deoxysphinganine. J. Biol. Chem. 2009;284:4786–4795. doi: 10.1074/jbc.M808798200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glenn A.E., Zitomer N.C., Zimeri A.M., Williams L.D., Riley R.T., Proctor R.H. Transformation-mediated complementation of a FUM gene cluster deletion in Fusarium verticillioides restores both fumonisin production and pathogenicity on maize seedlings. Mol. Plant Microbe Interact. 2008;21:87–97. doi: 10.1094/MPMI-21-1-0087. [DOI] [PubMed] [Google Scholar]
- 20.Guan X.L., Wenk M.R. Mass spectrometry-based profiling of phospholipids and sphingolipids in extracts from Saccharomyces cerevisiae. Yeast. 2006;23:465–477. doi: 10.1002/yea.1362. [DOI] [PubMed] [Google Scholar]
- 21.Fester T. Plant metabolite profiles and the buffering capacities of ecosystems. Phytochemistry. 2015;110:6–12. doi: 10.1016/j.phytochem.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 22.Folke C., Carpenter S., Walker B., Scheffer M., Elmqvist T., Gunderson L., Holling C.S. Regime shifts, resilience, and biodiversity in ecosystem management. Ann. Rev. Ecol. Evol. Syst. 2004;35:557–581. doi: 10.1146/annurev.ecolsys.35.021103.105711. [DOI] [Google Scholar]
- 23.Atanasova-Penichon V., Pons S., Pinson-Gadais L., Picot A., Marchegay G., Bonnin-Verdal M.N., Ducos C., Barreau C., Roucolle J., Sehabiague P., et al. Chlorogenic acid and maize ear rot resistance: A dynamic study investigating Fusarium graminearum development, deoxynivalenol production, and phenolic acid accumulation. Mol. Plant Microbe Interact. 2012;25:1605–1616. doi: 10.1094/MPMI-06-12-0153-R. [DOI] [PubMed] [Google Scholar]
- 24.Zitomer N.C., Riley R.T. Extraction and analysis of fumonisins and compounds indicative of fumonisin exposure in plant and mammalian tissues and cultured cells. Methods Mol. Biol. 2011;739:171–185. doi: 10.1007/978-1-61779-102-4_15. [DOI] [PubMed] [Google Scholar]
- 25.Wang H., Jones C., Ciacci-Zanella J., Holt T., Gilchrist D.G. Fumonisins and Alternaria alternata lycopersici toxins: Sphinganine analog mycotoxins induce apoptosis in monkey kidney cells. Proc. Natl. Acad. Sci. USA. 1996;93:3461–3466. doi: 10.1073/pnas.93.8.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helms J.B., Kaloyanova D.V., Strating J.R.P., van Hellemond J.J., van der Schaar H.M., Tielens A.G.M., van Kuppeveld F.J.M., Brouwers J.F. Targeting of the hydrophobic metabolome by pathogens. Traffic. 2015;16:439–460. doi: 10.1111/tra.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Desjardins A.E., Plattner R.D., Nelsen T.C., Leslie J.F. Genetic analysis of fumonisin production and virulence of Gibberella fujikuroi mating population A (Fusarium moniliforme) on maize (Zea mays) seedlings. Appl. Environ. Microbiol. 1995;61:79–86. doi: 10.1128/aem.61.1.79-86.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanchez-Rangel D., Sanchez-Nieto S., Plasencia J. Fumonisin B1, a toxin produced by Fusarium verticillioides, modulates maize beta-1,3-glucanase activities involved in defense response. Planta. 2011;235:965–978. doi: 10.1007/s00425-011-1555-0. [DOI] [PubMed] [Google Scholar]
- 29.Weber E., Bleiholder H. Explanations of the BBCH decimal codes for the growth stages of maize, rape, faba beans, sunflowers and peas—With illustrations. Gesunde Pflanzen. 1990;42:308–321. [Google Scholar]
- 30.Lancashire P.D., Bleiholder H., Boom T., Langeluddeke P., Stauss R., Weber E., Witzenberger A. A uniform decimal code for growth stages of crops and weeds. Ann. Appl. Biol. 1991;119:561–601. doi: 10.1111/j.1744-7348.1991.tb04895.x. [DOI] [Google Scholar]
- 31.Summerell B.A., Salleh B., Leslie J.F. A utilitarian approach to Fusarium identification. Plant Dis. 2003;87:117–128. doi: 10.1094/PDIS.2003.87.2.117. [DOI] [PubMed] [Google Scholar]
- 32.Raper K.B., Fennell D.I. In: The Genus Aspergillus. Robert E., editor. Krieger Publishing Company Inc.; Malabar, FL, USA: 1965. [Google Scholar]
- 33.Pitt J. The Genus Penicillium. Academic Press; London, UK: 1979. [Google Scholar]
- 34.Ludovici M., Ialongo C., Reverberi M., Beccaccioli M., Scarpari M., Scala V. Quantitative profiling of oxylipins through comprehensive LC-MS/MS analysis of Fusarium verticillioides and maize kernels. Food Addit. Contam. 2014;31:2026–2033. doi: 10.1080/19440049.2014.968810. [DOI] [PubMed] [Google Scholar]