FIGURE 6:
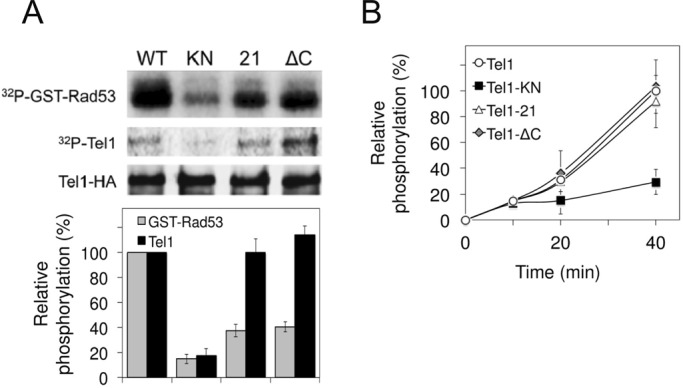
Effect of tel1-21 or tel1-ΔC mutation on protein kinase activity in vitro. (A) Effect of tel1-21 or tel1-∆C mutation on Rad53 phosphorylation and Tel1 autophosphorylation. Extracts were prepared from cells expressing Tel1-HA (KSC1709), Tel1-KN-HA (KSC1751), Tel1-21-HA (KSC3087), or Tel1-ΔC-HA (KSC3549) and subjected to immunoprecipitation with anti-HA antibodies. Immunoprecipitates were assayed for kinase reactions using GST-Rad53 C-terminus as a substrate. Top, Rad53 phosphorylation and Tel1 autophosphorylation were detected by autoradiography, and the amount of Tel1 protein was analyzed by immunoblotting with anti-HA antibodies. Bottom, phosphoincorporation was normalized relative to that from wild-type Tel1 protein (100%). The average intensity calculated from three independent experiments is shown, and the error bars represent the SD derived from these experiments. (B) Tel1 autophosphorylation after incubation for various lengths of time. Tel1 proteins were immunoprecipitated and subjected to the in vitro kinase assay as in A, but the reaction mixtures were incubated for various lengths of time (0, 10, 20, and 40 min). Phosphoincorporation into Tel1 was normalized relative to that for wild-type Tel1 protein at 0 (0%) and 40 min (100%).
