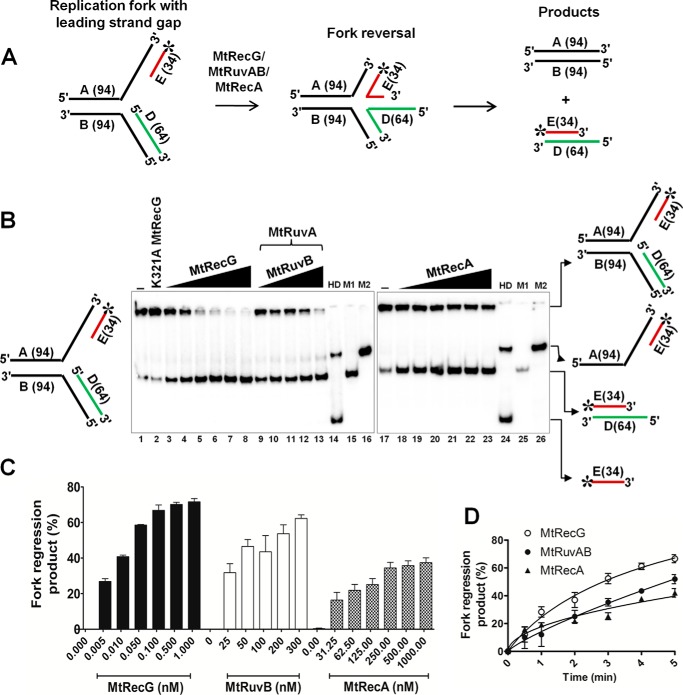
FIGURE 4.
Comparative fork reversal activities of M. tuberculosis RecG, RuvAB, and RecA with homologous replication fork containing a leading strand gap. A, schematic representation of homologous replication fork containing leading strand gap and outcome of fork reversal reaction. The asterisk indicates 32P label. B, analysis of RFR promoted by MtRecG, MtRuvAB, and MtRecA. Reaction mixture contained 10 nm 32P-labeled substrate in the presence of 1 mm ATP and 5 mm MgCl2. Reactions were initiated by addition of the following: 0.005, 0.01, 0.05, 0.1, 0.5, and 1.0 nm MtRecG (lanes 3–8, respectively);50 nm MtRuvA, 25, 50, 100, 200, and 300 nm MtRuvB (lanes 9–13, respectively); and 31.25, 62.5, 125, 250, 500, and 1000 nm MtRecA (lanes 18–23, respectively). Lanes 14 and 24 represent heat-denatured substrate. Lanes 15 and 25 (M1) denote a marker of leading and lagging strand annealed products, respectively. Lanes 16 and 26 (M2) represent a marker for products that are generated by helicase unwinding of the parental strands. Lanes 1 and 17 denote reactions in the absence of any protein. Lane 2 represents reaction with 10 nm K321A MtRecG. C, quantitative data for the efficiency of MtRecG-, MtRuvAB-, and MtRecA-catalyzed fork reversal. D, kinetics of fork reversal activities of MtRecG, MtRuvAB, and MtRecA proteins. The reactions were initiated by addition of 0.1 nm MtRecG, 100/300 nm MtRuvA/MtRuvB, and 250 nm MtRecA in a reaction buffer containing 10 nm substrates with 1 mm ATP and 5 mm MgCl2. Reactions were terminated at indicated time intervals, and products were resolved on 8% native polyacrylamide gel and analyzed by autoradiography. The error bars represent standard error of the mean (S.E.) from three independent experiments.