FIGURE 7.
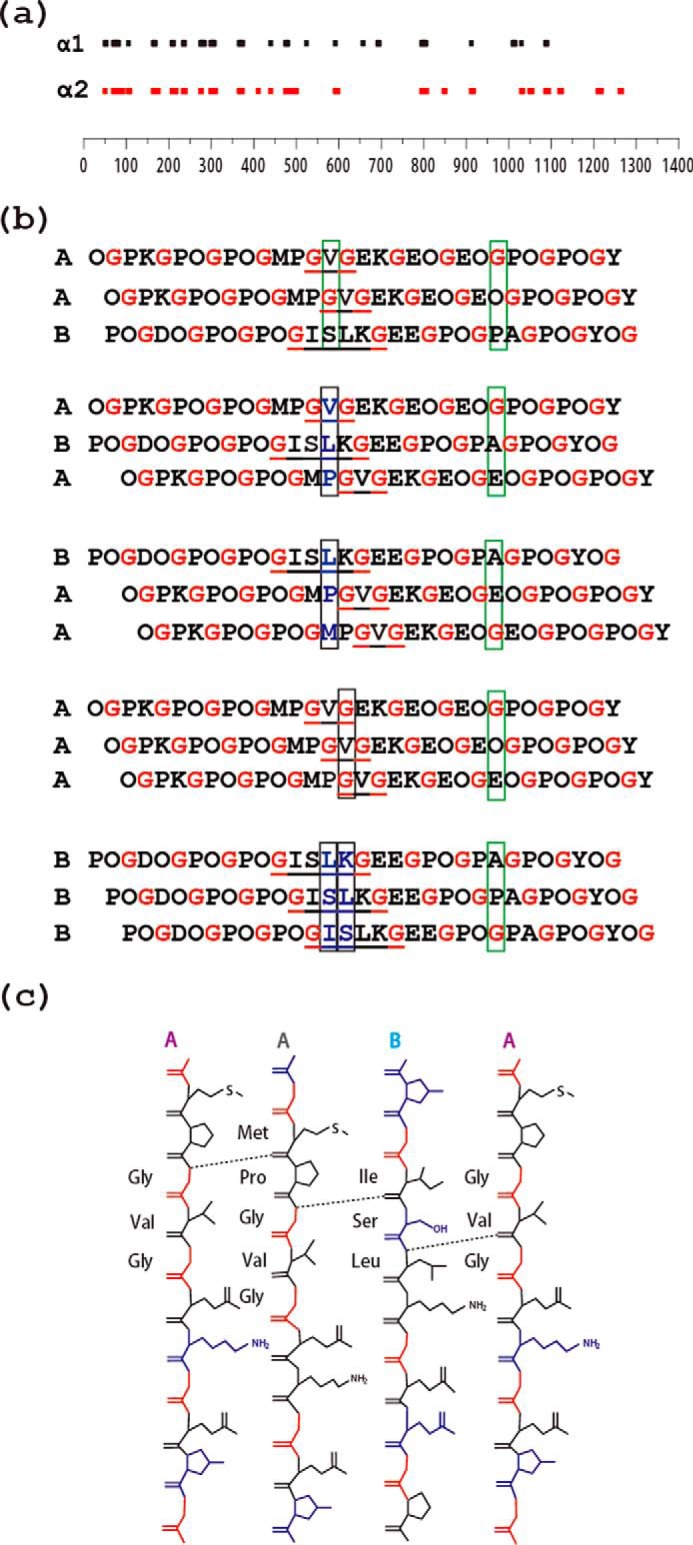
a, schematic diagram of the location of interruptions along the α1(IV) (top) and α2(IV) (bottom) chains of human type IV collagen. The lengths of the bars are proportional to the number of amino acids in the interruption sequence. The alignment illustrates that many, but not all, interruptions are at the same sites in the two chains within a [α1(IV)]2α2 triple-helix molecule, whereas the lengths of the interruptions often differ between the two chains at a given site. b, schematic illustration of the effect of the staggering between the three chains within a triple-helix on interchain hydrogen bonding at the interruption site. The three possible registers for the A and B peptides are shown on the top (AAB, ABA, BAA), whereas homotrimers AAA and BBB are shown at the bottom. The presence of a one-residue GVG interruption in two chains and a four-residue GISLKG interruption in the other two chains allows maintenance of the stagger on both sides of the interruption (commensurate). Gly residues are shown in red to visualize the stagger, and interruptions are underlined. Black boxes are used to designate an axial level where there is no Gly and the typical stabilizing interchain Gly NH … CO bonds cannot be formed. Green boxes are used to exemplify a regular axial level that contains one and only one Gly with the expected formation of a hydrogen bond. Only AAB allows hydrogen bonding at all axial positions, as proposed by Bella (46). c, hydrogen bonding at the interruption site GVG/GVG/ISLK in heterotrimer AAB deduced from amide temperature-dependent NMR studies, with hydrogen bonds formed between the amide group of Gly14/Leu16 and the carbonyl group of nearby amino acids indicated as dashed lines.
