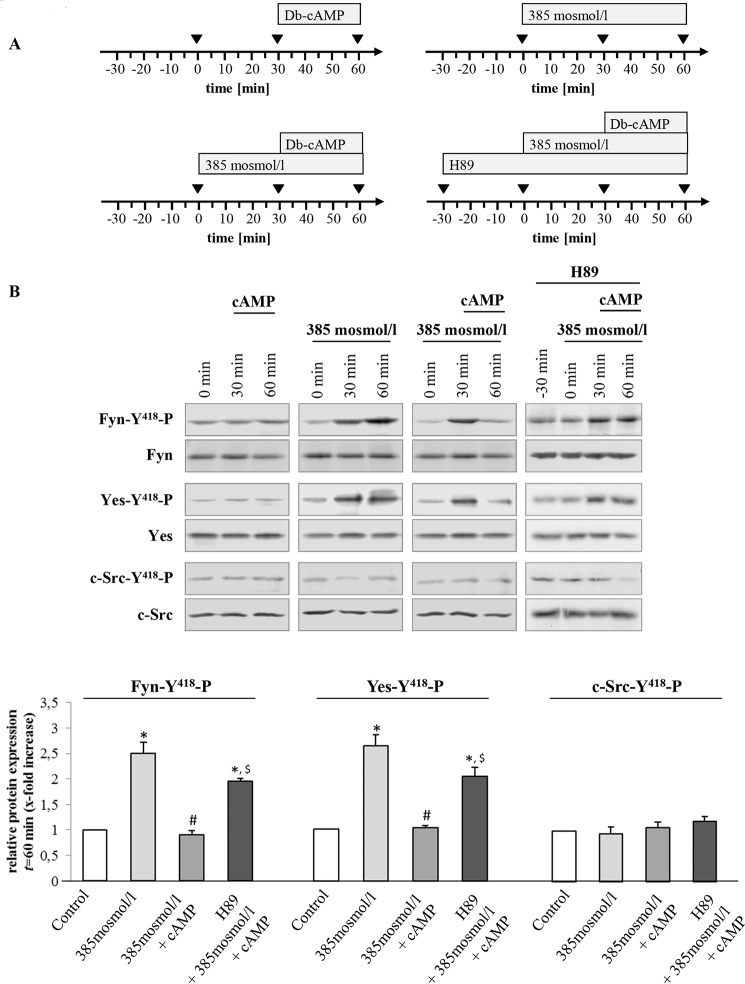
FIGURE 7.
Bt2cAMP triggers PKA-dependent inhibition of hyperosmolarity-induced Src kinase family activation. Rat livers were perfused as described under “Experimental Procedures” and as mentioned in the perfusion plans (A) of the respective experiments. B, liver samples were analyzed for activation of Src kinase family members Fyn, Yes, and c-Src. Blots were analyzed densitometrically. t = 60 min was compared with the respective control (t = 0 min, set as 1), i.e. sample without institution of either hyperosmolarity (385 mosmol/liter) or Bt2cAMP (50 μmol/liter) or with H89 (2 μmol/liter). Representative blots and densitometric analysis (means ± S.E.) of at least three independent perfusion experiments are shown. In line with Fig. 5, hyperosmolarity induced within 30 min a significant phosphorylation of Fyn and Yes (*, p < 0.05), although no c-Src activation was observed. Phosphorylation of Fyn and Yes was inhibited by Bt2cAMP (#, p < 0.05). Inhibition of Fyn and Yes phosphorylation by Bt2cAMP was sensitive to H89 ($, p < 0.05).