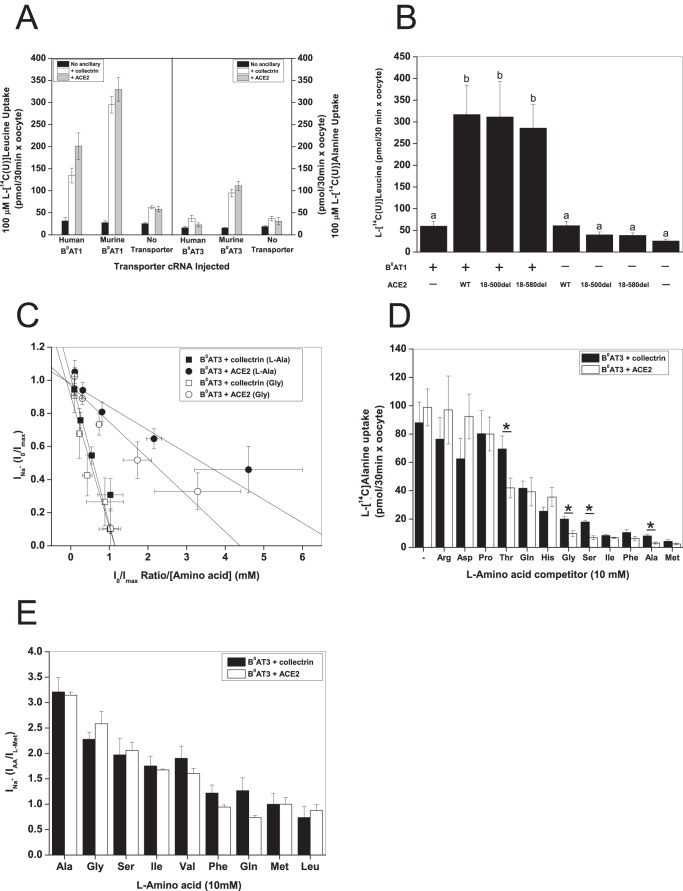
FIGURE 1.
Ancillary proteins modify substrate affinity of B0AT1 and B0AT3. X. laevis oocytes were injected with 10 ng of the indicated transporter, 2 ng of collectrin, and 10 ng of ACE2 cRNA in the indicated combinations except where otherwise stipulated. All uptake and electrophysiological measurements were made day 4–6 post-injection. A, uptake of 100 μm l-[U-14C]leucine or 100 μm l-[U-14C]alanine was measured in oocytes expressing B0AT1 or B0AT3 over 30 min. Each bar represents mean ± S.D. (n = 12, e > 3. B, B0AT1 was coexpressed with truncated (18–500del or 18–580del) or wild-type ACE2. Uptake was measured as in A (n = 10−12, e = 3). a and b above the individual bars indicate groupings of conditions whose differences of means are not statistically significant from each other at the p = 0.05 level. C, oocytes were voltage-clamped at −50 mV and then perfused with serial concentrations of l-alanine. Data were transformed according to Eadie-Hofstee and analysed by linear regression, for all regressions 0.99 > R2 > 0.88, and Pearson's r > −0.94 (n = 7). D, oocytes were injected with 6 ng of B0AT3 cRNA, 5 ng of ACE2, and 2 ng of collectrin cRNA. Uptake was measured as in A with the exception that it was challenged by 10 mm unlabeled l-amino acids as indicated. The first pair of bars from the left represents unchallenged 100 μm l-[U-14C]alanine uptake. l-[U-14C]Alanine uptake from uninjected oocytes was subtracted. Each bar represents mean ± S.D. (n = 8−10, *, p < 0.05). E, oocytes were perfused with 10 mm of all the amino acids indicated on the abscissa, and subsequent steady-state Na+ currents were recorded. All currents were normalized to the maximal current induced by 10 mm l-methionine. Each bar represents mean ± S.D. (n = 6, e = 3).