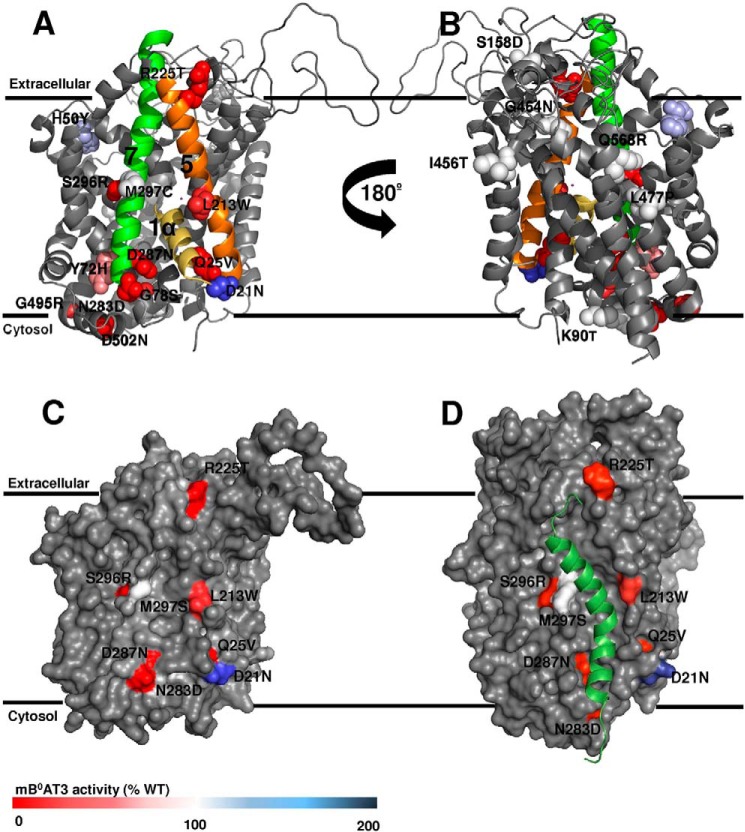
FIGURE 4.
Surface localization of B0AT3 mutants displayed on a homology model. Transport activity of all mutants is indicated by color coding and was determined by flux assays in X. laevis oocytes as described in Fig. 1. Data were taken from Table 2. The homology model structure of mouse B0AT3 was built using the D. melanogaster dopamine transporter DAT x-ray crystal structure in the outward open confirmation (PDB 4m48) (A–C) or LeuT from A. aeolicus in an outward occluded confirmation (PDB 2A65) (D) as a template. Mouse B0AT3 is viewed parallel to the membrane. Mutated residues are shown in van der Waals representation. Wild-type activity is 100% (white); lower activity is displayed in red, and higher activity in blue. A, transmembrane helices 1α, 5, and 7 are colored yellow, orange, and green, respectively. B, homology model is rotated 180° on its vertical axis. C, surface representation of B0AT3 in the same orientation as shown in A. The pocket is flanked by TM domains 5 and 7 and partially occupied by TM1α. Only mutants located in, or peripheral to, TM1α, TM5, or TM7 are labeled. D, hypothesized interaction site between collectrin's TM domain (Met-136–Arg-171) and B0-like transporters. The model is based on the end point of a 10-ns MD simulation (see “Experimental Procedures”). The same face of mouse B0AT3 is shown as in other panels. The transporter is viewed parallel to the membrane with the POPC membrane used during MD simulation removed to see the proteins clearly. Mouse B0AT3 is visualized in gray with collectrin Met-136 to Arg-171 in dark green. The same residues are shown as in C.