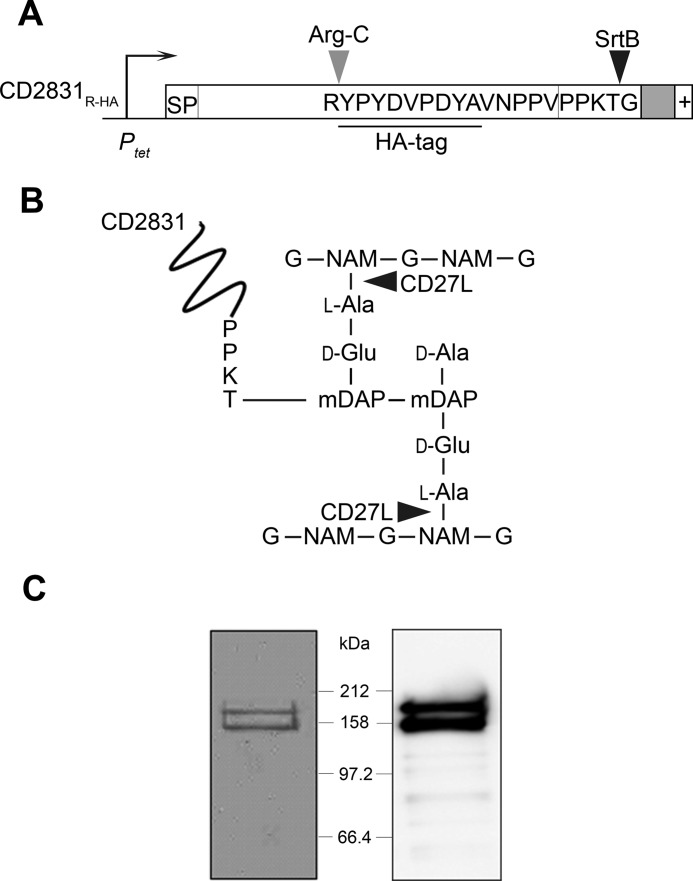
FIGURE 5.
Expression and purification of CD2831R-HA from the cell wall of C. difficile ΔzmpI. A, CD2831R-HA construct under control of the inducible tetracycline promoter (Ptet) containing signal peptide (SP), the PPKTG motif, the hydrophobic domain (in gray), and the positively charged tail (+). Cleavage sites of SrtB and ArgC are indicated. B, CD2831 is covalently linked to the peptidoglycan of C. difficile. Nonacetylated glucosamine residues (G) and unusual mDAP-mDAP cross-links generated by dl-transpeptidation are predominant in the peptidoglycan of C. difficile (34). Cleavage sites of the CD27L endolysin are indicated by arrowheads. NAM, N-acetylmuramic acid; DAP, diaminopimelic acid. C, cells from ΔzmpI strain expressing CD2831R-HA were suspended in PS buffer and treated with the purified catalytic domain of the CD27L endolysin. Digested cell wall was isolated by centrifugation and subjected to immunoprecipitation using anti-HA. Purified polypeptides were separated on 10% SDS-polyacrylamide gels and Coomassie Blue-stained (left panel) or probed with anti-HA antibodies (right panel).