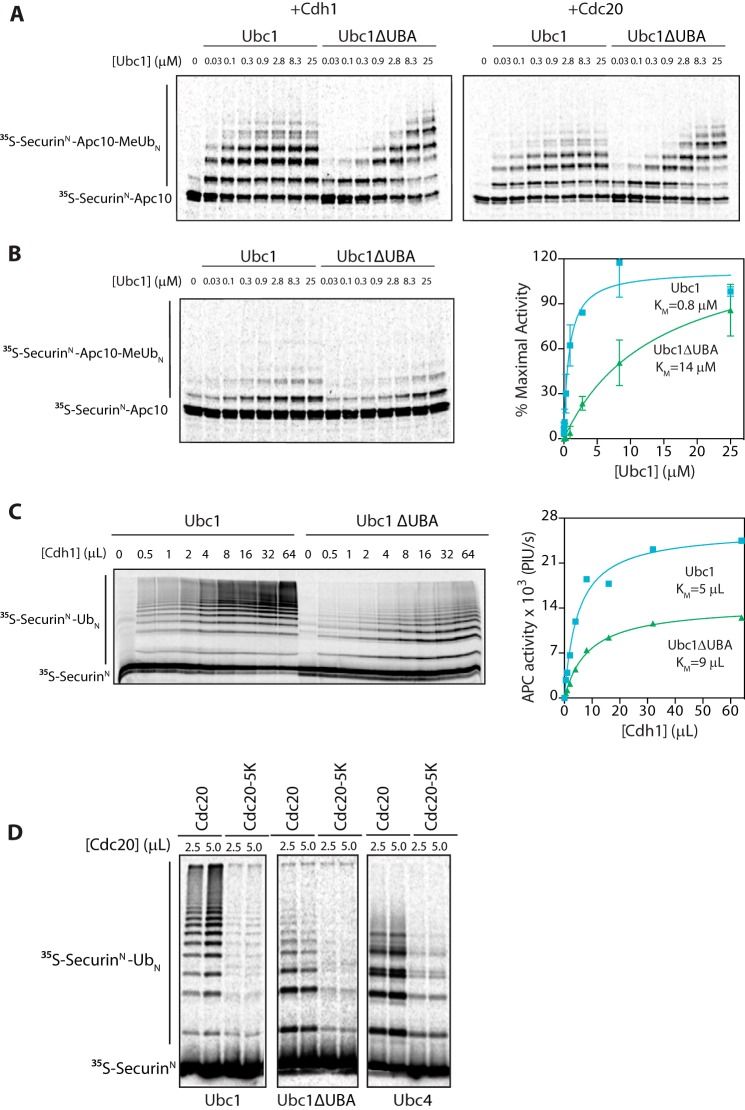
FIGURE 3.
UBA domain does not bind to APC/C activator. A, [35S]SecurinN-Apc10, Cdh1, and Cdc20 were generated by in vitro translation in rabbit reticulocyte lysate. [35S]SecurinN-Apc10 was bound to immunoprecipitated APC/C (apc10Δ cdh1Δ), and unbound substrate was washed away. Cdh1 and Cdc20 were purified from reticulocyte lysate and mixed with fusion substrate-bound APC/C. Ubc1 (either wild type or ΔUBA) charged with methylated ubiquitin (MeUb) was added in increasing concentrations, and reactions were carried out at room temperature for 15 min. Reaction products were analyzed by SDS-PAGE and autoradiography with a phosphorimaging system. Results are representative of three independent experiments. B, APC/C assays were performed as in A except that no activator subunit was added. The graph at right shows mean values ±S.E. normalized to maximal Ubc1 activity of three independent experiments. C, [35S]SecurinN and Cdh1 were generated by in vitro translation in rabbit reticulocyte lysate and purified. Immunoprecipitated APC/C was mixed with [35S]SecurinN and increasing amounts of Cdh1. Ubc1 or Ubc1ΔUBA (10 μm) was charged with wild-type ubiquitin (Ub), and reactions were carried out at room temperature for 15 min. Reaction products were analyzed as in A. The graph at right shows quantification of APC/C activity as a function of Cdh1 concentration for the experiment at left. Results are representative of three independent experiments. PIU/s, phosphorimaging units/s. D, [35S]SecurinN, Cdc20, and Cdc20-5K were generated as in C and mixed with purified APC/C. E2s (each at 5 μm) charged with wild-type ubiquitin were added, and reactions were carried out at room temperature for 30 min. Reaction products were analyzed as in A. Results are representative of three independent experiments. Error bars represent S.E.