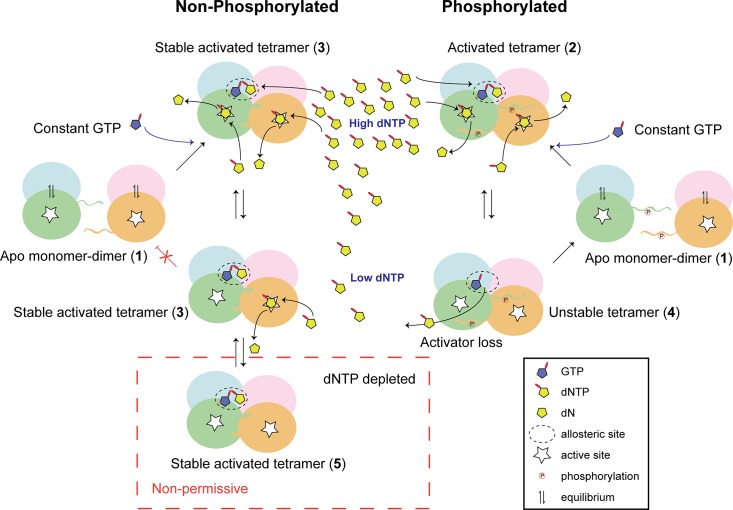
Fig 8. Model for phospho-regulation of SAMHD1 restriction.
In the absence of dNTPs Apo-SAMHD1 is found in a monomer-dimer equilibrium regardless of the phosphorylation state (1). At high dNTP levels, typically in cycling cells, constitutively abundant GTP combines with dNTPs to fill allosteric sites. In both phosphorylated and un-phosphorylated SAMHD1 this results in the formation of an activated tetramer (2) that in the non-phosphorylated protein also includes additional intra-tetramer CtD interactions forming a stable activated tetramer (3). Under these conditions, both activated and stable activated tetramers hydrolyse the dNTP pool at comparable rates. At lower dNTP levels, the stabilisation afforded by the CtD interactions maintains enzyme activity in non-phosphorylated SAMHD1 by preventing the loss of dNTP-activator from the allosteric site. However, in phospho-SAMHD1, activating dNTPs dissociate from the allosteric site (4) resulting in disassembly of the tetramer and down-regulation of triphosphohydrolase activity. At very low levels, such as in differentiated myeloid cells, CtD-stabilised tetramers still retain activating dNTPs in the allosteric site (5) and SAMHD1 remains catalytically competent. It can therefore rapidly respond to any increase in intracellular dNTPs to maintain the dNTP levels below the threshold required for HIV-1 replication.