Abstract
Background
Pachyonychia congenita (PC) is a rare autosomal dominant disease whose main clinical features include hypertrophic onychodystrophy and palmoplantar keratoderma. The new classification is based on genetic variants with mutations in keratin KRT6A, KRT6B, KRT6C, KRT16, KRT17, and an unknown mutation. Here, we present a case of PC with unusual clinical and histological features and a favorable response to oral acitretin.
Case
A 49-year-old male presented with diffuse and striate palmoplantar keratoderma, thickened nails, knuckle pads, and pseudoainhum. Histology showed compact hyperkeratosis, prominent irregular acanthosis, and extensive epidermolytic hyperkeratosis, suggestive of Vörner's palmoplantar keratoderma. However, keratin 9 and 1 were not mutated, and full exome sequencing showed heterozygous missense mutation in type I keratin K16.
Conclusion
To our knowledge, epidermolytic hyperkeratosis has not been previously described with PC. Our patient had an excellent response, maintained over the last 5 years, to a low dose of acitretin. We wish to emphasize the crucial role of whole exome sequencing in establishing the correct diagnosis.
Key Words: Pachyonychia congenita, Palmoplantar keratoderma, Exome sequencing
Introduction
Pachyonychia congenita (PC) is a rare autosomal dominant disorder. Its main features, reported in almost 90% of the patients, include hypertrophic onychodystrophy and palmoplantar keratoderma [1]. Oral leukokeratosis, hoarseness, natal teeth, and epidermoid cysts are seen less frequently. The most distressing symptom is severe plantar pain likely due to the development of blisters under the hyperkeratosis [2]. The old classification (types I and II) has been replaced by a new classification based on the following genetic mutations discovered in 1995: PC-K6a, PC-K6b, PC-K6c, PC-K16, PC-K17, and PC-U, which stand for mutations in keratin KRT6A, KRT6B, KRT6C, KRT16, KRT17, and an unknown mutation, respectively [3]. Mutation in keratin 16 is the second most common mutation in PC after K6a, based on a survey of 523 individuals from the International PC Research Registry [4]. Almost all patients with a mutation in K16 have thickened toenails (96%), plantar keratoderma (99%), and plantar pain (97%), while 78% display palmar keratoderma. Oral leukokeratosis (40%), cysts (27%), and follicular hyperkeratosis (14%) are less commonly described in patients with PC-K16. Natal or prenatal teeth, a feature of PC-K17, have not been reported in patients with PC-K16.
Herein, we present a case with debilitating palmoplantar keratoderma that showed prominent epidermolytic hyperkeratosis on histology. It was initially thought to represent a severe case of Vörner's palmoplantar keratoderma until whole exome sequencing clinched the diagnosis of PC. This patient had additional unusual clinical features and a favorable response to oral acitretin.
Case Report
A 49-year-old French-Canadian male presented to our outpatient clinic with thick hyperkeratosis of the palms and soles. He reported that his paternal great-grandfather, two of his brothers, and his daughter had or have similar but milder lesions. This condition became apparent at the age of 6 months and gradually worsened over the years. The associated deep fissures made standing, walking and using his hands very difficult and painful. The pain and functional limitations were especially severe in the morning, when the patient had to crawl to the bathroom on his knees, leaning on the dorsum of his hands. Over the years, he had learned to regularly pare the thickened keratin off his soles and to use over-the-counter emollients that he found more effective than prescribed keratolytic preparations.
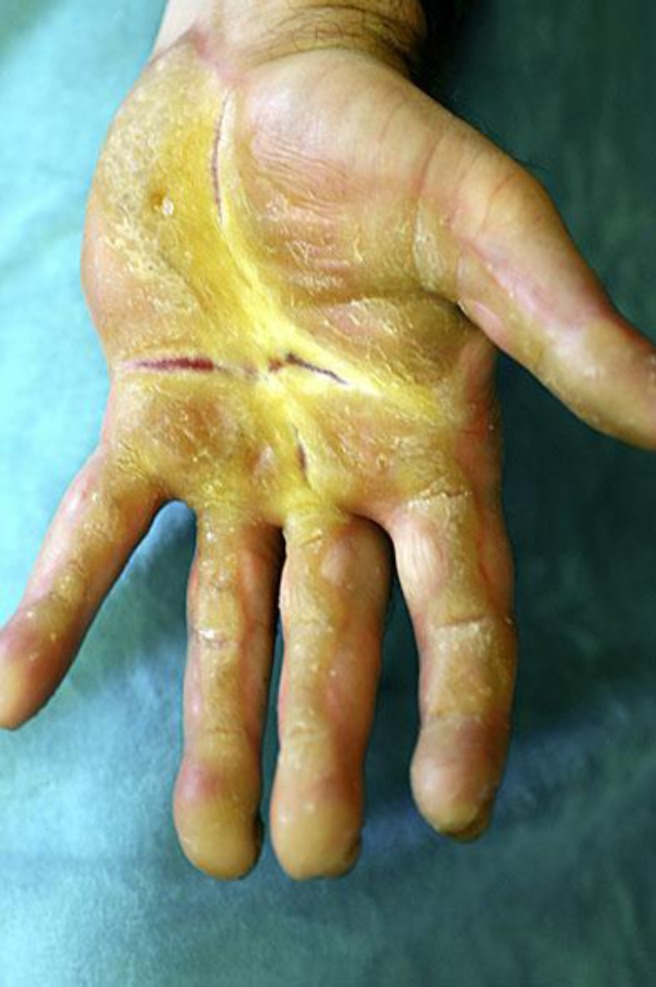
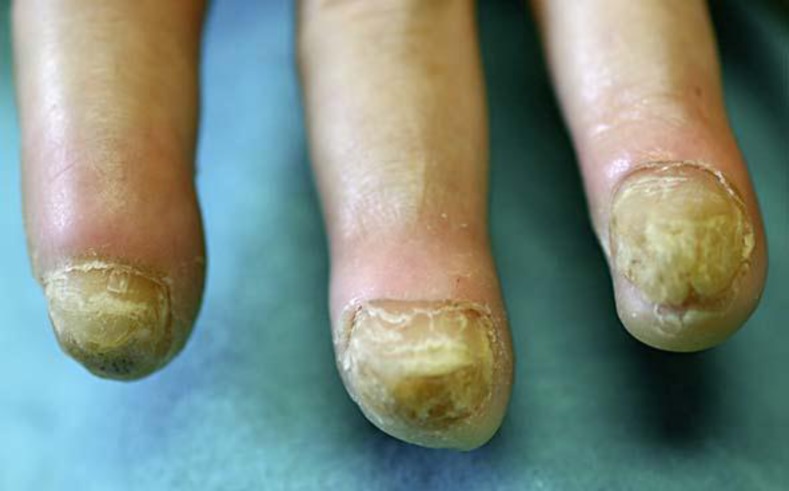
Examination revealed compact, thick, and yellow palmoplantar hyperkeratosis with deep fissuring most prominent in the center of both palms. The keratoderma was surrounded with well-defined erythematous borders. The soles were diffusely thickened, while on the palms, the thenar and hypothenar areas were relatively spared. The hyperkeratosis involved the volar aspect of the fingers in a wavy linear pattern (fig. 1). The hyperkeratosis did not extend over the dorsum of the hands and feet, and no starfish keratosis was seen. Knuckle pads were noted over the metacarpophalangeal articulations, and the fingertips were pointed and sclerotic. The toes were twisted and swollen, and three of them were encircled at their base with constriction bands (pseudoainhum). The nails were grayish, grossly thickened, and showed a markedly increased longitudinal curvature described as ‘parrot-beaking’ (fig. 2). There was no involvement of the hair and teeth, the oral mucosa was normal as was the voice, no epidermal cyst was seen, and the general physical examination was normal.
Fig. 1.
Severe and fissured palmar keratoderma with a wavy, linear pattern on the volar aspect of the fingers.
Fig. 2.
Thickened and friable nails with increased longitudinal and transverse curvatures.
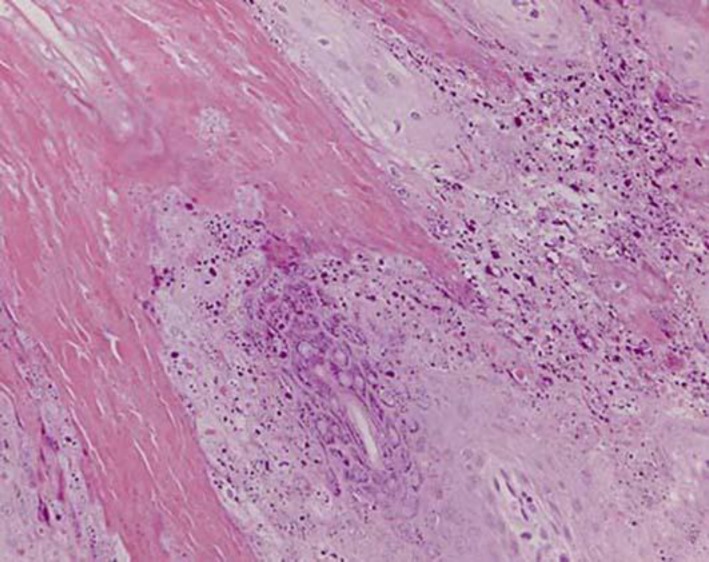
A 4-mm punch biopsy showed massive, compact hyperkeratosis with parakeratosis, prominent irregular acanthosis, and extensive epidermolytic hyperkeratosis (granulovacuolar degeneration of the keratinocytes; fig. 3). The patient and his daughter, similarly affected, were referred for molecular testing and genetic counseling. Sequencing of exon 1 of the KRT9 gene and select exon analysis of the KRT1 gene failed to reveal mutations in keratin 1 and keratin 9. A final diagnosis of PC was established when whole exome sequencing was subsequently carried out and showed that both the patient and his daughter harbored a heterozygous mutation in KRT16 (L124R). No other pathogenic mutation was found.
Fig. 3.
Compact hyperkeratosis with parakeratosis, acanthosis, and prominent epidermolytic hyperkeratosis (hematoxylin and eosin stain).
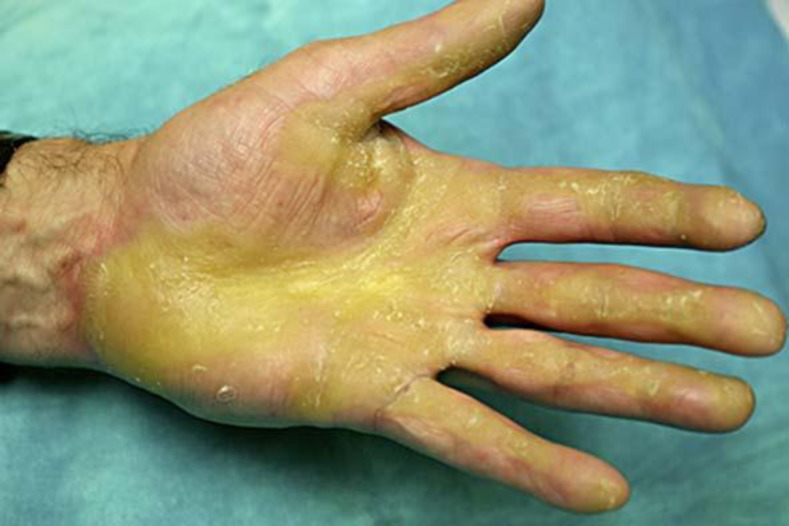
The patient was treated with acitretin 10 mg once a day for 1 month and tolerated the medication well. The dose was then increased to twice a day. He responded well, with a reduction of the hyperkeratosis in both palms and soles, healing of the fissures (fig. 4), loosening of the pseudoainhum, as well as with improved mobility and dexterity, which have been maintained over the last 5 years.
Fig. 4.
Healing of fissures and reduction of the hyperkeratosis after 6 months of treatment with acitretin 20 mg daily.
The daughter is less severely affected, presenting with thickened nails, diffuse plantar keratoderma, and focal palmar hyperkertosis in a striate pattern reminiscent of her father's, although without fissuring or limitation in mobility. Given her desire to raise a family, she declined treatment with acitretin.
Discussion
Our patient presented with a constellation of clinical signs, some being rather unusual: (1) a striate pattern of palmar hyperkeratosis together with diffuse involvement of the soles; (2) thickening of the nails, initially embedded in the massive plantar hyperkeratosis, but better revealed when the keratoderma had improved with acitretin; (3) knuckle pads, most likely an epiphenomenon resulting from friction when the patient was crawling on the dorsum of his hands, which regressed when he became more mobile, and (4) the formation of constriction bands without autoamputation of the toes. This finding has recently been reported in a case of Vörner-Unna-Thost palmoplantar keratoderma with mutation in keratin 9 [5].
The severity of the clinical manifestations, combined with the histopathological findings of extensive epidermolytic hyperkeratosis initially led us toward a diagnosis of Vörner's palmoplantar keratoderma. However, sequencing of exon 1 of the KRT9 gene and select exon analysis of the KRT1 gene failed to reveal mutations in keratin 1 and keratin 9. Full exome sequencing [6] was instrumental in uncovering a heterozygous missense mutation in type I keratin K16 and clearly established the diagnosis of PC.
A mutation in L124R of KRT16 has already been described in a father and his son [7]. Their presentation was similar to our case with regard to severe thickening of the nails and an absence of oral lesion. However, the keratoderma in our case was diffuse as compared to the focal involvement in the reported cases. More recently, a novel heterozygous missense mutation (p.Leu421Pro) of KRT16 was discovered in a large Spanish family [8]. Affected members exhibited a mild phenotype of PC with focal non-epidermolytic palmoplantar keratoderma and thick nails. Phenotypic heterogeneity is common among PC patients and correlates with abnormalities in specific keratins. It has been shown that patients with KRT16 mutations have more extensive and painful palmoplantar keratoderma while those with KRT6A mutations have more severe nail disease, oral lesions, cysts, and follicular hyperkeratosis [9]. Furthermore, different mutations within the KRT16 gene can also result in phenotype variations [10]. The total number of reported keratin mutations in PC now stands at 105, 20 of which involve KRT16 [11].
Histologically, our patient displayed prominent granulovacuolar keratinocyte degeneration (epidermolytic hyperkeratosis), a feature not, to our knowledge, previously reported in PC and that led to a delay in diagnosis. However, it affected our therapeutic approach. We felt that our patient could benefit from systemic retinoids, but, at the same time, we were aware that this treatment could increase his skin fragility and worsen his symptoms. We elected to begin treatment with a daily dose of 10 mg of acitretin, which was well tolerated. The dose was increased to 20 mg daily after 1 month and maintained over the past 5 years. The nail changes remained relatively unaffected, but there was rapid healing of fissures, approximately 50% reduction in thickness of the keratoderma, and a tremendous increase in quality of life in terms of regained mobility and regression of pain. In contrast, the cases reported by Smith et al. [7] showed no response to 25–50 mg of acitretin for several months. A recent study evaluated the effectiveness and adverse effects of isotretinoin, etretinate, and acitretin in 30 patients with PC [12]. Fifteen patients (50%) reported thinning of the hyperkeratosis, 10 patients (33%) had decreased pain, but 8 (27%) complained of increased pain. The daily doses of retinoids ranged from 10 to 50 mg. Patients receiving low doses (<25 mg/day) reported better effectiveness and greater satisfaction than those treated with higher doses [12]. The overall effectiveness was 58% for acitretin and 36% for isotretinoin [12].
In conclusion, we present a case of PC-K16 with unusually severe palmoplantar keratoderma and pseudoainhum, atypical features not usually seen in PC. The histological features of epidermolytic hyperkeratosis are unique and, to our knowledge, have not been previously described with PC. Our patient had an excellent response to a low dose of acitretin, which appears to be the treatment of choice for this condition. We wish to emphasize the crucial role of whole exome sequencing, a novel technique that allowed the precise establishment of the diagnosis in this kind with peculiar clinical and histological characteristics. This tool opens up new horizons in the exploration of the multiple phenotypic variants of numerous genodermatoses by allowing the discovery and mapping of a large array of known and new mutations.
Statement of Ethics
The patient and his daughter gave informed consent to genetic testing, including full exam sequencing. They also consented to have their clinical photographs used for publication purposes. Given that they were not participating in a study, their cases were not submitted to our Institutional Review Board.
Disclosure Statement
The authors report no conflicts of interest.
References
- 1.Eliason MJ, Leachman SA, Feng BJ, Schwartz ME, Hansen CD. A review of the clinical phenotype of 254 patients with genetically confirmed pachyonychia congenita. J Am Acad Dermatol. 2012;67:680–686. doi: 10.1016/j.jaad.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg I, Sprecher E, Schwartz ME, Gaitini D. Comparative study of high-resolution multifrequency ultrasound of the plantar skin in patients with various types of hereditary palmoplantar keratoderma. Dermatology. 2013;226:365–370. doi: 10.1159/000351321. [DOI] [PubMed] [Google Scholar]
- 3.McLean WH, Hansen CD, Eliason MJ, Smith FJ. The phenotypic and molecular genetic features of pachyonychia congenita. J Invest Dermatol. 2011;131:1015–1017. doi: 10.1038/jid.2011.59. [DOI] [PubMed] [Google Scholar]
- 4.International Pachyonychia Congenita Research Registry (IPCRR) Data Summary (May 9, 2014). http://www.pachyonychia.org.
- 5.Funakushi N, Mayuzumi N, Sugimura R, Ikeda S. Epidermolytic palmoplantar keratoderma with constriction bands on bilateral fifth toes. Arch Dermatol. 2009;145:609–610. doi: 10.1001/archdermatol.2009.83. [DOI] [PubMed] [Google Scholar]
- 6.Majewski J, Schwartzentruber J, Lalonde E, Montpetit A, Jabado N. What can exome sequencing do for you? J Med Genet. 2011;48:580–589. doi: 10.1136/jmedgenet-2011-100223. [DOI] [PubMed] [Google Scholar]
- 7.Smith FJ, Fisher MP, Healy E, Rees JL, Bonifas JM, Epstein EH, Jr, et al. Novel keratin 16 mutations and protein expression studies in pachyonychia congenita type 1 and focal palmoplantar keratoderma. Exp Dermatol. 2000;9:170–177. doi: 10.1034/j.1600-0625.2000.009003170.x. [DOI] [PubMed] [Google Scholar]
- 8.Paris F, Hurtado C, Azón A, Aguado L, Vizmanos J. A new KRT16 mutation associated with a phenotype of pachyonychia congenita. Exp Dermatol. 2013;22:832–849. doi: 10.1111/exd.12262. [DOI] [PubMed] [Google Scholar]
- 9.Spaunhurst KM, Hogendorf AM, Smith FJD, Lingala B, Schwartz ME, Cywinska-Bernas A, Zeman KJ, Tang JY. Pachyonychia congenita patients with mutations in KRT6A have more extensive disease compared with patients who have mutations in KRT16. Br J Dermatol. 2012;166:875–878. doi: 10.1111/j.1365-2133.2011.10745.x. [DOI] [PubMed] [Google Scholar]
- 10.Fu T, Leachman SA, Wilson NJ, Smith FJ, Schwartz ME, Tang JY. Genotype-phenotype correlations among pachyonychia congenita patients with K16 mutations. J Invest Dermatol. 2011;131:1025–1028. doi: 10.1038/jid.2010.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson NJ, O'Toole EA, Milstone LM, Hansen CD, Shepherd AA, Al-Asadi E, Schwartz ME, McLean WHI, Sprecher E, Smith FJD. The molecular genetic analysis of the expanding pachyonychia congenita case collection. Br J Dermatol. 2014;171:343–355. doi: 10.1111/bjd.12958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gruber R, Edlinger M, Kaspar RL, Hansen CD, Leachman S, Milstone LM, Smith FJD, Sidoroff A, Fritsch PO, Schmuth M. An appraisal of oral retinoids in the treatment of pachyonychia congenita. J Am Acad Dermatol. 2012;66:e193–e199. doi: 10.1016/j.jaad.2011.02.003. [DOI] [PubMed] [Google Scholar]